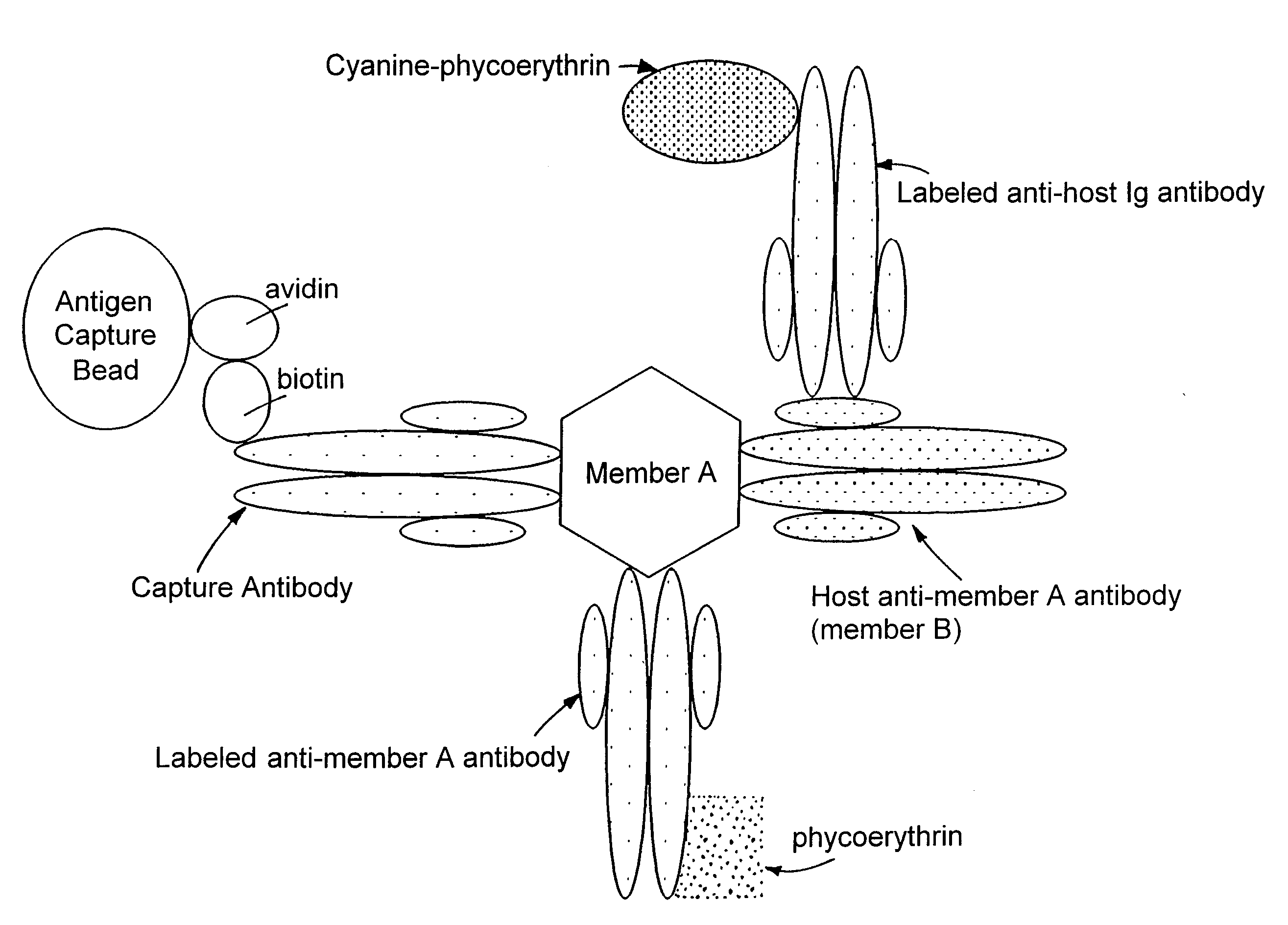
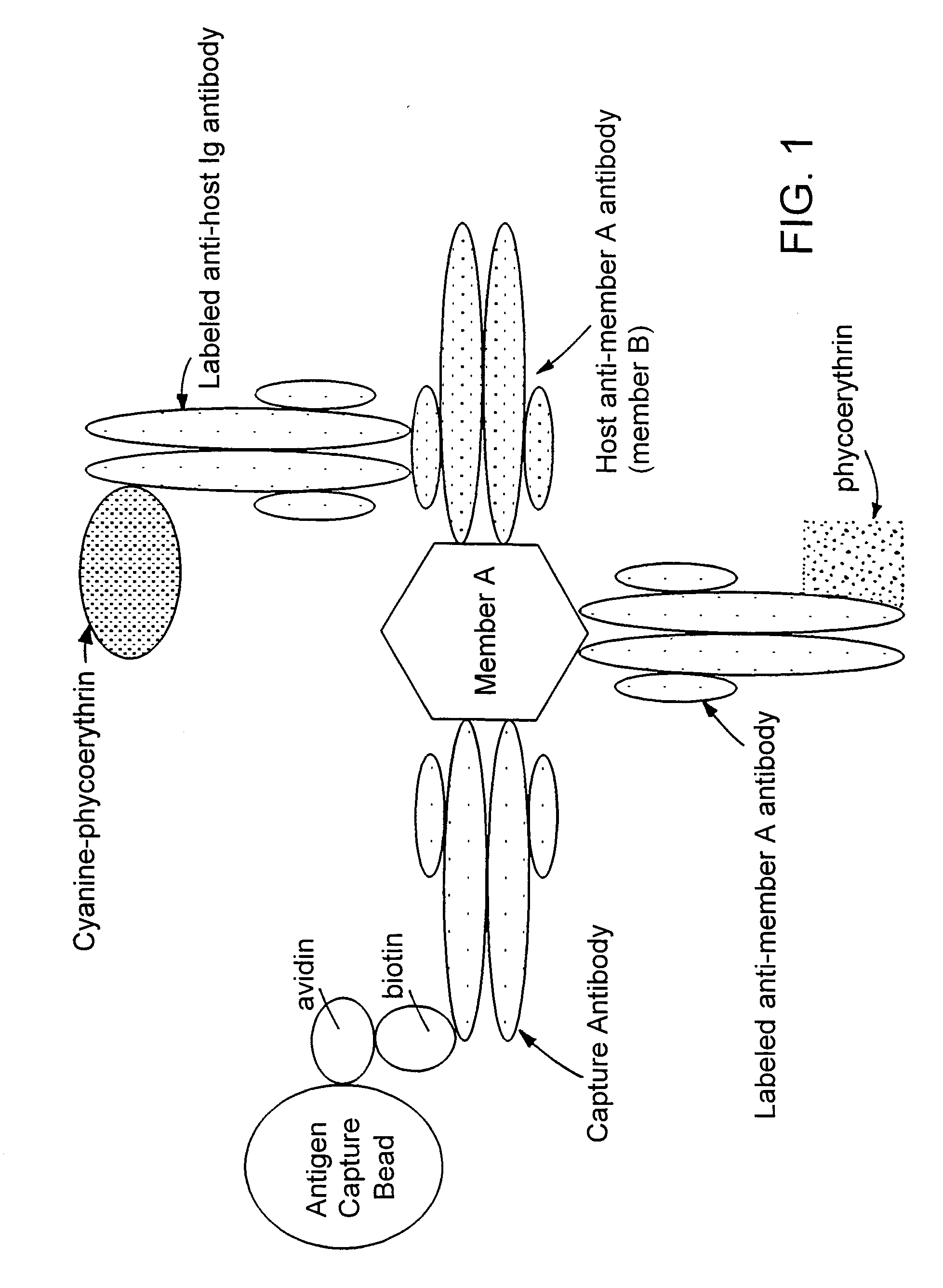
Methods for simultaneously detecting both members of a binding pair
a technology of binding pair and detection method, which is applied in the field of simultaneous detection of both members of a binding pair, can solve the problems of reducing the detection efficiency of antibodies, so as to enhance the ability to detect infections and improve the detection efficiency. the effect of rapid and sensitiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0036]Biotinylation of Proteins: Antibodies were conjugated at a concentration of 5 mg / ml; viral antigens were conjugated at a concentration of 1 mg / ml. To biotin label anti-viral protein antibodies and viral antigens, the proteins were exchanged into 100 mM KH2CO3 buffer (pH 8.3) using an appropriate size Centricon (Amicon) filter.
[0037]Biotin N-hydroxysuccinimidyl ester (Molecular Probes, Eugene, Oreg.) in DMSO (10 mg / ml, Sigma Chemical Co., St. Louis, Mo., Cat. #D8779) was prepared immediately prior to use, and added to the protein to be biotinylated in a 5:1 or 10:1 molar ratio. Reactions were performed by vortexing the protein solution lightly, and adding the biotin / DMSO to the protein solution and mixing thoroughly. Protein and biotin ester were reacted for one hour at room temperature in the dark. Conjugated protein was separated from free biotin by separation on a 10 ml Sephadex-25 gel column or spin column using 1×PBS to elute. Individual 1 ml fractions were collected and a...
example 2
[0039]Production of Analyte Capture Beads: Analyte capture beads were prepared by completely resuspending avidin-coated paramagnetic beads (7 μm, Spherotech, VM-60-100) by mixing well. Beads (typically 3.8×106 beads) were placed in a 50 ml centrifuge tube and mixed with 30 ml of 1×PBS. After retaining beads on the side of the tube with magnets, all PBS was removed. The beads were washed two more times with PBS.
[0040]After the final PBS wash, the required volume of biotinylated antibody (typically 40 μg) and 2 ml of 1×PBS were added to the beads. The beads were resuspended by vortexing continuously for a minimum of 3 hours, or by vortexing for one hour and then storing overnight at 4° C. Beads stored overnight were vortexed for an additional 2 hours the next morning. Approximately 30 ml of buffer containing 1% FBS and 0.1% NaN3 in PBS were used to wash the conjugated beads three times. Beads were resuspended in 19.25 ml of the same buffer and stored at 4° C. until use.
[0041]To label ...
example 3
[0042]Detection of Viral Antigens and Host Antibody: Plasma samples from normal individuals and from individuals positive for HCV, HIV, or HBV were obtained from New York Biologicals (Southampton, N.Y.), Scantibodies Laboratory (Santee, Calif.), or Intergen BioDiagnostics (Purchase, N.Y.). Plasma samples were treated with Triton-X 100 detergent to a final concentration of 0.5% to lyse viral membranes and expose core particles prior to testing. E. coli derived recombinant viral antigens, including surface and core antigens, were obtained from BiosPacific (Emeryville, Calif.) or Intergen BioDiagnostics. Antigens were added to normal non-pathologic serum samples for development of a reference standard curve and for use in spike and recovery analysis.
[0043]Flow cytometric analysis was performed on a Coulter EPICS Profile TI, a Coulter XL, or a Partec PAS flow cytometer using linear forward vs. side light scatter to gate the bead population. Fluorescence signal was amplified logarithmica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| sizes | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com