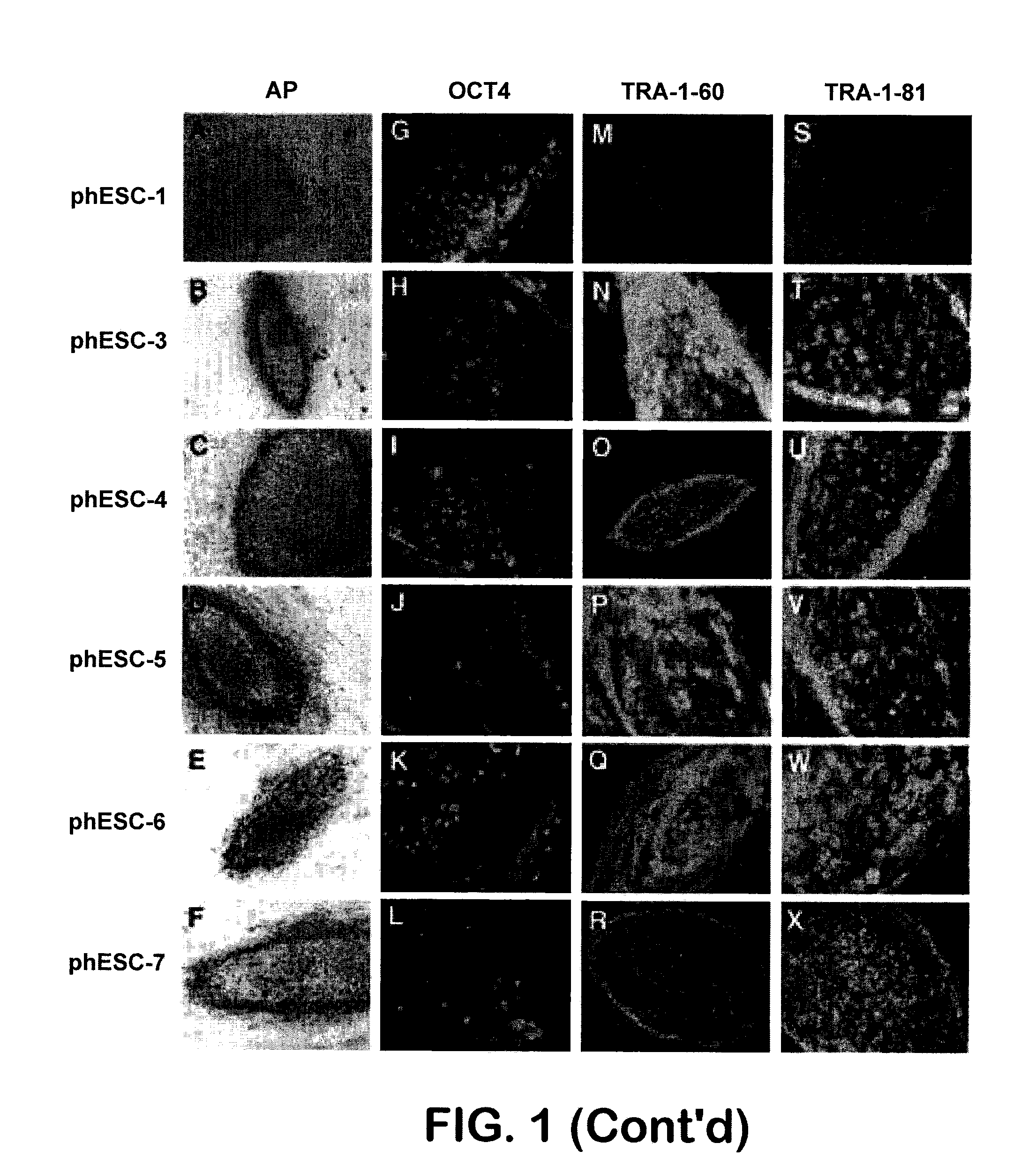
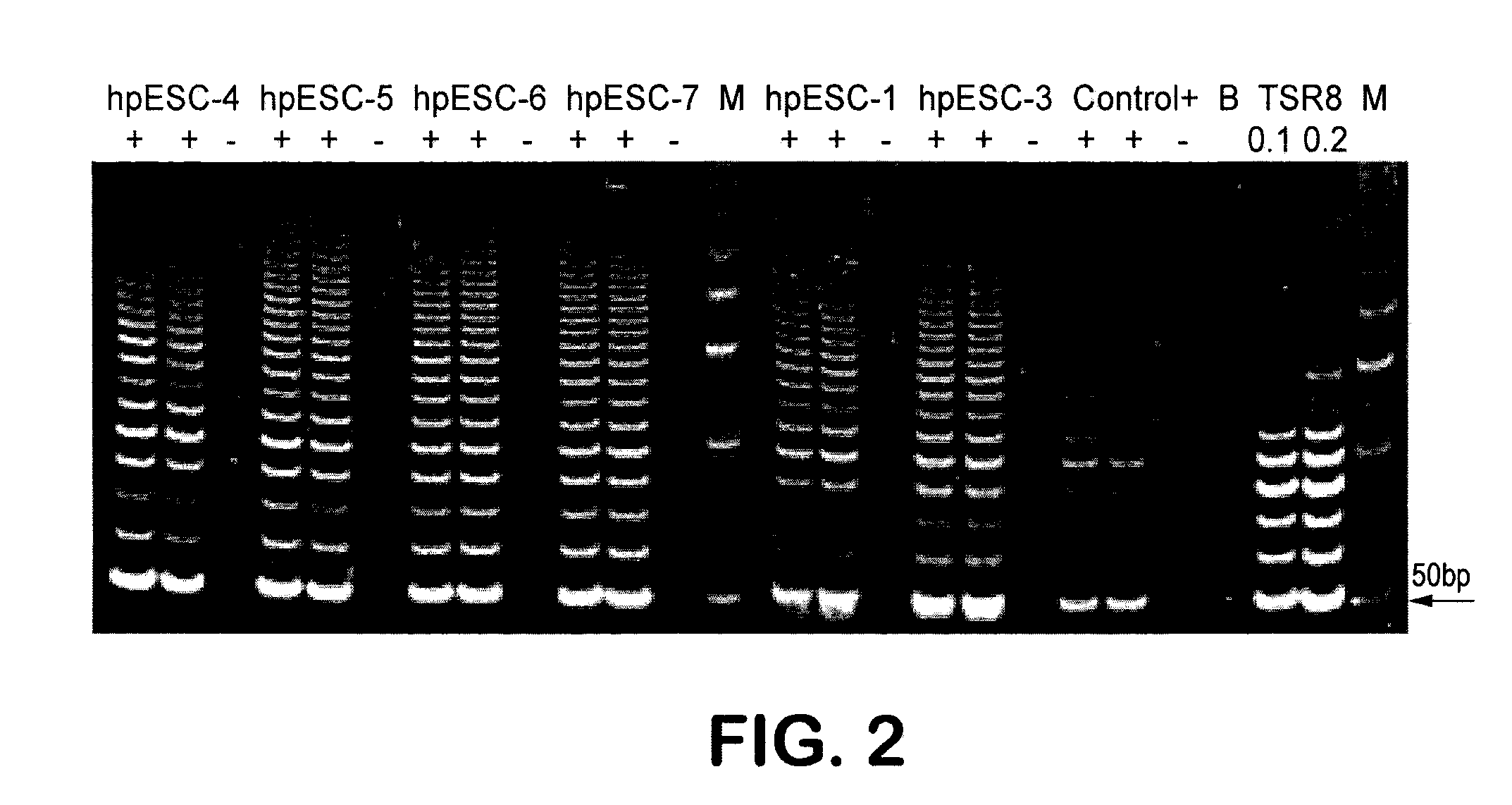
Patient-specific stem cell lines derived from human parthenogenetic blastocysts
a technology of parthenogenetic blastocysts and stem cells, applied in the field of embryonic stem cells, can solve problems such as teratoma formation, and achieve the effect of minimizing the use of animal-derived cells and high levels of alkaline phosphatase and telomerase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of Parthenotes
[0203]Five oocyte donors, all over 31 years of age, participated in this study. Oocytes were obtained using hormonal stimulation with the primary intent of in vitro fertilization (IVF). A total of 46 cumulus-oocyte complexes (COCs) were taken from five donors and used for this study (Table 1).
TABLE 1Generation of parthenotes and parthenogenetic embryonic stem cell lines.Blastocystsderived6withoutOocytesOocytesOocytesParthenoteswithvisibleDonorDonorderiveddonatedactivatedcreated5ICMICMLines generateddestiny18 4 442—phESC-1pregnantimmunosurgery215 8 8833phESC-3pregnantphESC-4twinsphESC-5all from wholeblastocysts32714141,a1132phESC-6pregnantfrom wholeblastocyst422111131023phESC-7 frompregnantwholeblastocyst520 94 7714no cell linenotgeneratedpregnant1two oocytes were not activated;2one oocyte degenerated after activation;3one oocyte was not activated;4two oocytes were at metaphase I stage and were discarded;5total pathenogenetically activated oocytes = 40;6tota...
example 2
Derivation of an hpSC-Hhom Line from an HLA Homozygous Donor
[0221]With an initial goal of isolating an HLA homozygous parthenogenetic human stem cell line, oocytes from an HLA homozygous donor were used. HLA genotyping of both the donor (donor 1) and her parents demonstrated that both parents were heterozygous. The same haplotype A*25, B*18, DRB1*15 was inherited from each parent, with the donor having an HLA homozygous genotype A*25, A*25, B*18, B*18, DRB1*15, DRB1*15 (Table 5, Case 1).
[0222]Nineteen cumulus-oocyte complexes (COCs) were taken from donor 1, of which seven were used for research (Table 4).
TABLE 4Origin of parthenotes andHLA homozygous parthenogenetic human stem cell lines.DonorOocytesOocytesBlasto-NumberharvesteddonatedcystsCell linesIVF Result11974hpSC-Hhom-1Successful21873hpSC-Hhom-2SuccessfulhpSC-Hhom-3(Twinpregnancy)3201000Unsuccessful427142hpSC-Hhom-4SuccessfulTotal843894NA
[0223]Parthenogenetic activation was performed using a previously described protocol with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com