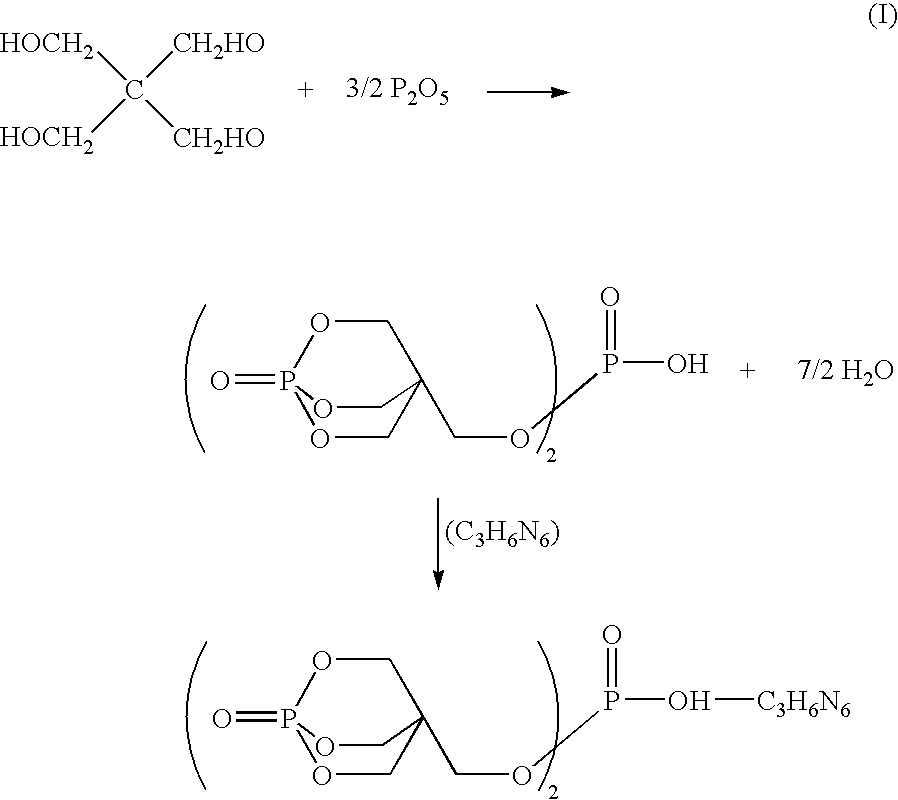
Method for preparing melamine salt of bis(pentaerythritol phosphate) phosphoric acid
a technology of pentaerythritol phosphate and melamine salt, which is applied in the field of preparing a melamine salt of bis (pentaerythritol phosphate) phosphoric acid, can solve the problems of numerous limitations on the selection of reaction solvents, the inability to recollect phosphorus oxychloride liquid, and the high molar ratio of excessive phosphorus oxychloride to be used for the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0019]Diphosphorous pentaoxide (P2O5) and pentaerythritol (PE) were fed into a double-screw extruder at a molar ratio of 1:1. Esterification was performed at a rotation speed of 100 min−1 and a sleeve temperature in the range of 30 to 200° C. to obtain bis(pentaerythritol phosphate) phosphoric acid. Sampling was made at the outlet of the extruder, and a 31P-NMR map was used to calculate the esterifying rate. The result was recorded in Table 1.
[0020]An amount of 122.4 g of bis(pentaerythritol ester) phosphate, 111 g of melamine, and 500 g of pure water was added into a 1000 mL beaker, and the reacting temperature was increased to 90° C. while stirring. The duration of the process was 30 minutes. Then, the reacting temperature was decreased to 25° C. to remove water by filtration. A melamine salt of bis(pentaerythritol phosphate) phosphoric acid was obtained after baking.
example 2-3
[0021]Steps in EXAMPLE 1 were repeated, and the screw rotation speed of the double-screw extruder was adjusted to carry out esterification according to Table 1. Sampling was made at the outlet of the extruder, and a 31P-NMR map was used to calculate the esterifying rate. The results were recorded in Table 1.
example 4
[0022]Steps in EXAMPLE 1 were repeated, and the screw rotation speed of the double-screw extruder was adjusted to carry out esterification according to Table 1. Sampling was made at the outlet of the extruder, and a 31P-NMR map was used to calculate the esterifying rate. The result was recorded in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com