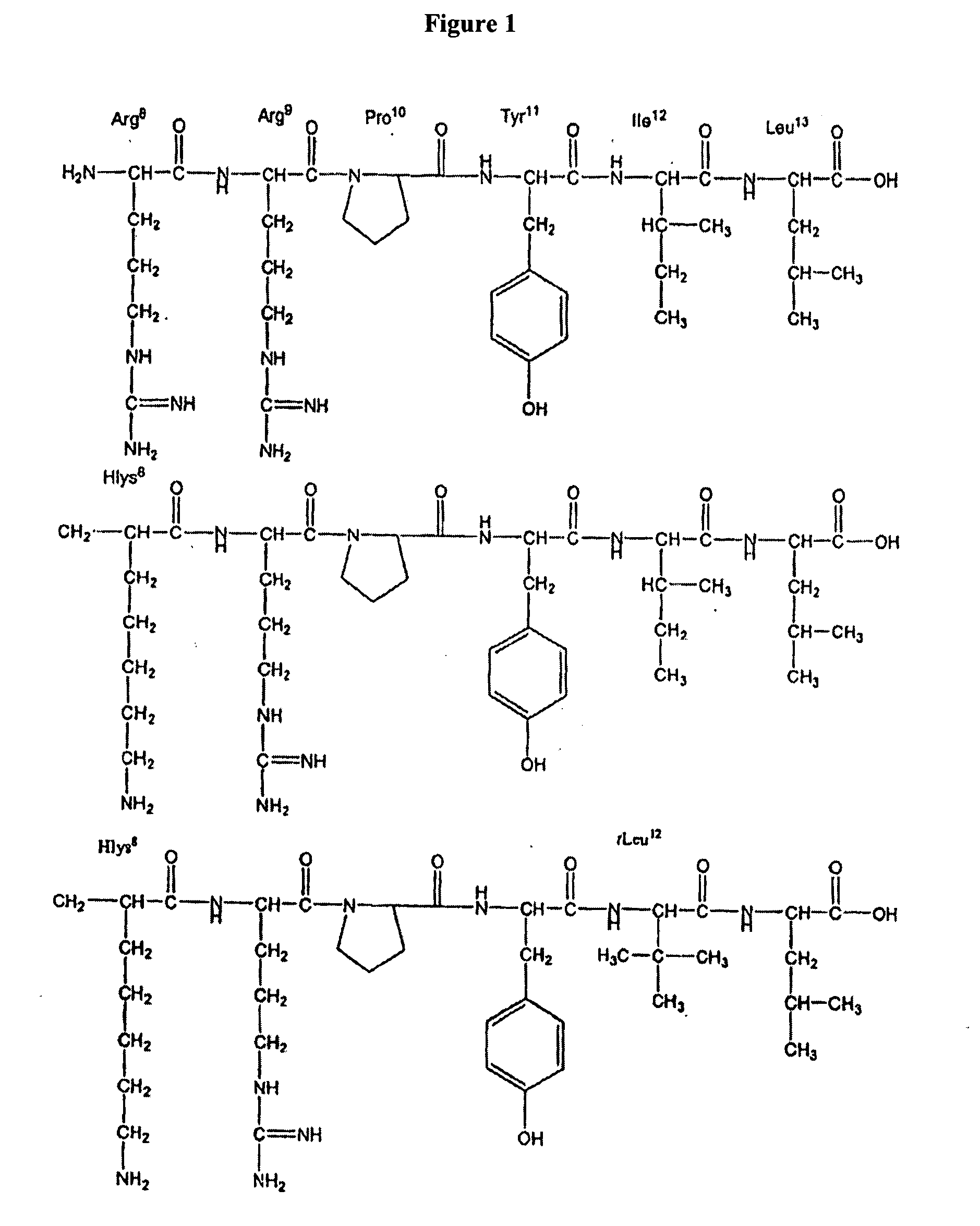
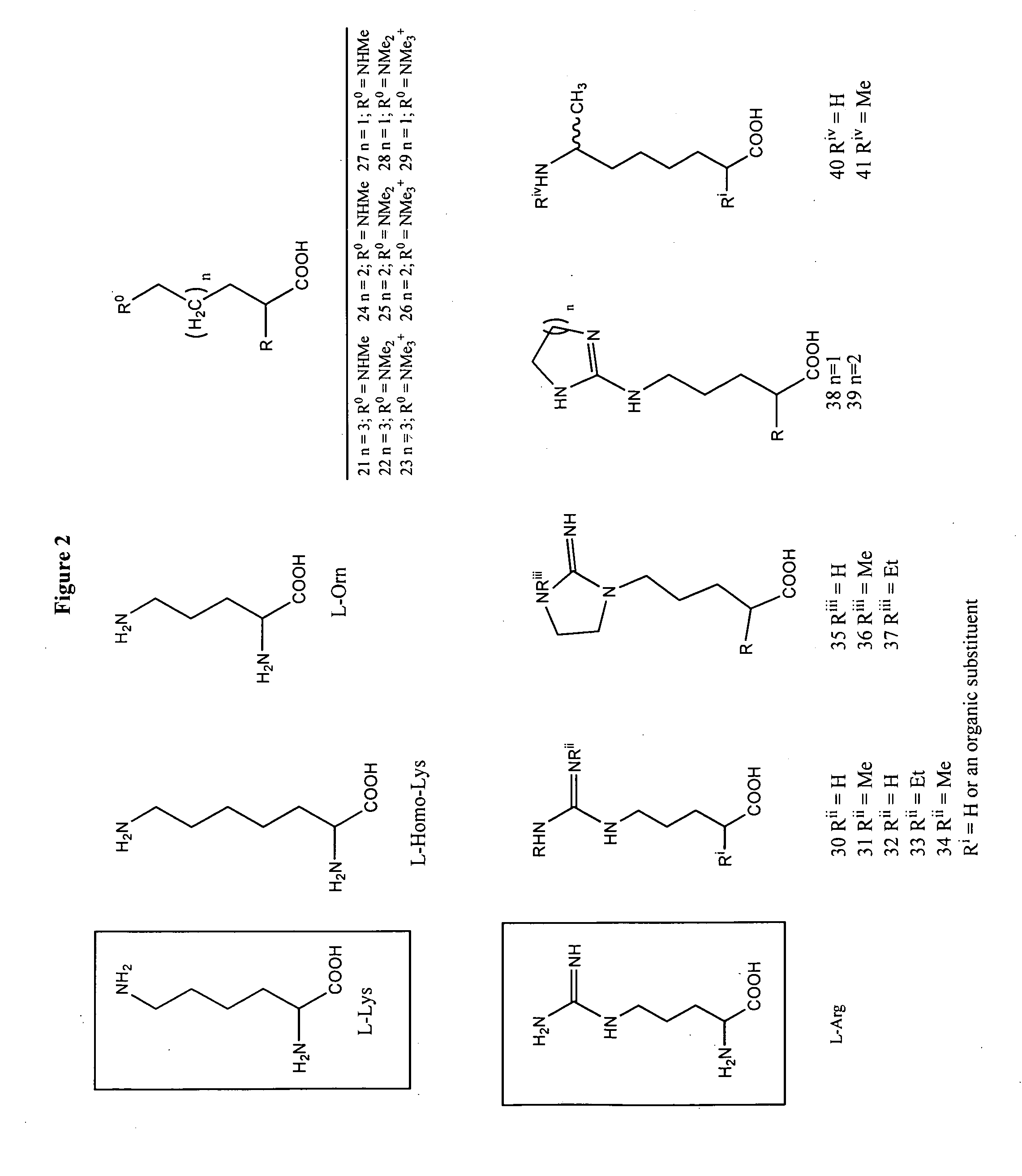
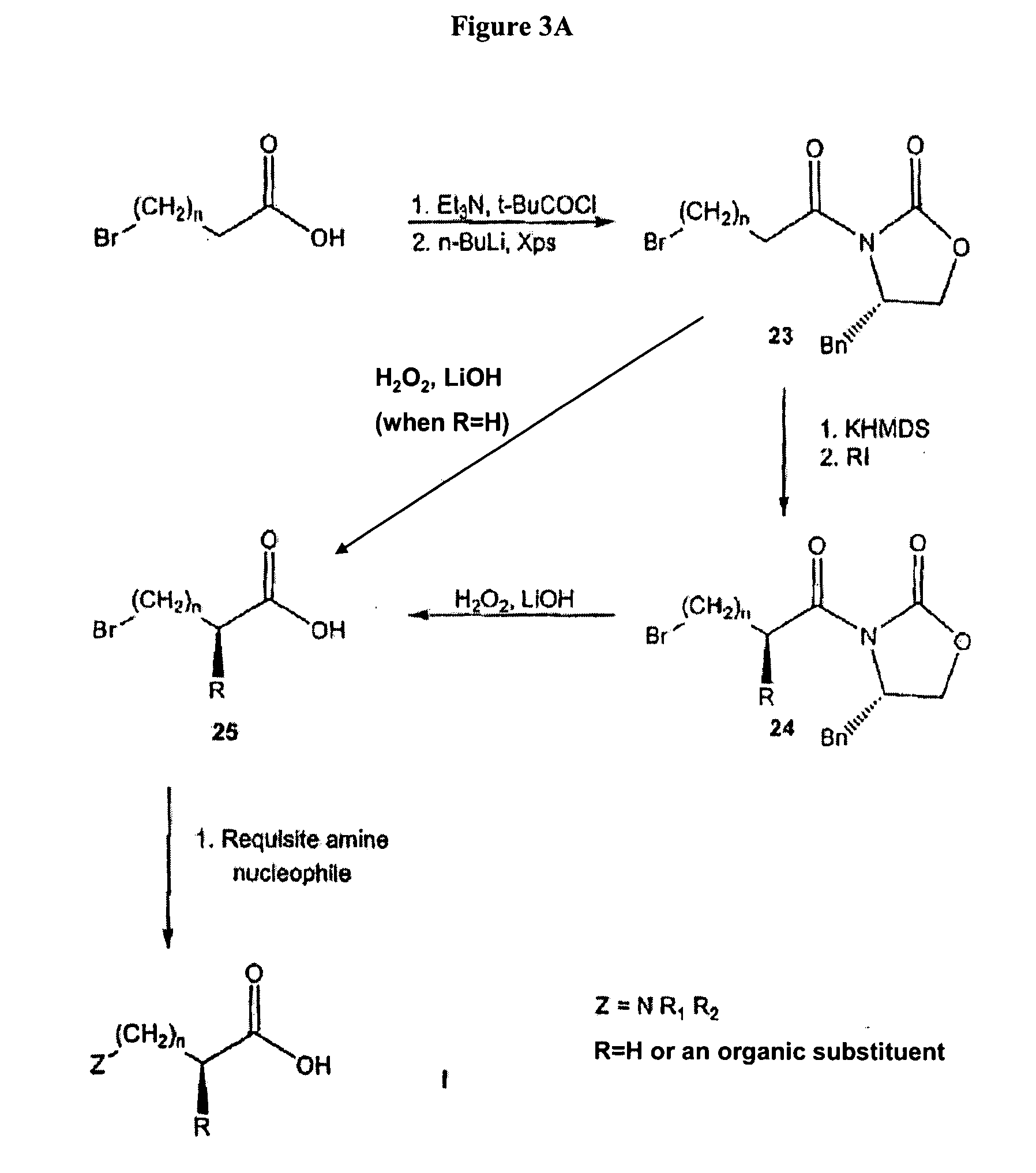
Non-natural amino acids and neurotensin analogues
a technology of amino acids and neurotensins, which is applied in the field of non-natural amino acids and neurotensin analogues, can solve the problems of poor drug candidates, rare selectivity required of viable drug candidates, and most peptides are unable to cross biological membranes, and achieves selective and long-lasting biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Summary of Neurological Effects of the NT Peptides of the Invention
[0485]The N-terminal alpha methyl, alpha desamino homolysyl and orinthyl analogues of NT(8-13) prepared according to the invention (see the foregoing general discussion and the Examples) were synthesized and screened for activity in numerous behavioral assays predictive of antipsychotic potential. These peptides induced hypothermia in a dose-dependent fashion after oral administration. In addition, oral administration of the peptides significantly reduced d-amphetamine induced hyperlocomotion, a measure of the therapeutic efficacy of current or potential APDs. The low dose of peptide (10 mg / kg) that elicits a significant response after oral administration in these assays is significant. The peptides also demonstrate an ability to maintain efficacy after repeated administration. In fact they demonstrate an ability to increase maximal hypothermic response over time, implying that repeated administration may actually im...
example 2
Compound Synthesis
[0505]The following examples and protocols are put forth so as to provide those of ordinary skill in the art with a complete disclosure and description of how the compounds claimed herein are made and evaluated, and are intended to be purely exemplary of the invention and are not intended to limit the scope of what the inventors regard as their invention. Efforts have been made to ensure accuracy with respect to numbers (e.g., amounts, temperature, etc.) but some errors and deviations should be accounted for. Unless indicated otherwise, parts are parts by weight, temperature is in ° C. and is at room temperature, and pressure is at or near atmospheric.
[0506]Starting Materials. Solvents are from Fisher Scientific (Pittsburgh, Pa.) and reagents from Aldrich (Milwaukee, Wis.) unless otherwise noted.
[0507]Abbreviations. Trisyl-N3, 2,4,6-triisopropylbenzenesulfonyl azide; Et3N, triethylamine; t-BuCOCl, trimethylacetylchloride; n-BuLi, n-butyl lithium; H2, hydrogen gas; ...
example 3
Alpha methyl, Alpha desamino, omega N-substituted homolysyl and orinthyl (8) neurotensin (8-13)
[0514]Alpha methyl, alpha desamino omega N-substituted homo lysyl and orinthyl (8) neurotensin (8-13) were synthesized (FIG. 7). The α-methyl bromo acids, 27a and c, were coupled to the resin-bound peptide as outlined in the general section. The solid state coupling was conducted as follows.
[0515]Resin bound N alpha Fmoc leucine was swelled in DMF prior to Fmoc cleavage with piperidine (20% in DMF). The piperidine solution was removed with vacuum filtration and the resin-bound amino acid washed with DMS and methylene chloride (5× each). Amino acids (4 eq) were activated in DMF with HOBt (4 eq) PyBOP ((4 eq) and DIPEA (10 eq) and added directly to the peptide reaction vessel. Amino acids were coupled for 6 hours, the resins was washed with DMF and methylene chloride and monitored with a Kaiser test for the presence of free amines. Residues were recoupled when necessary. This procedure was r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com