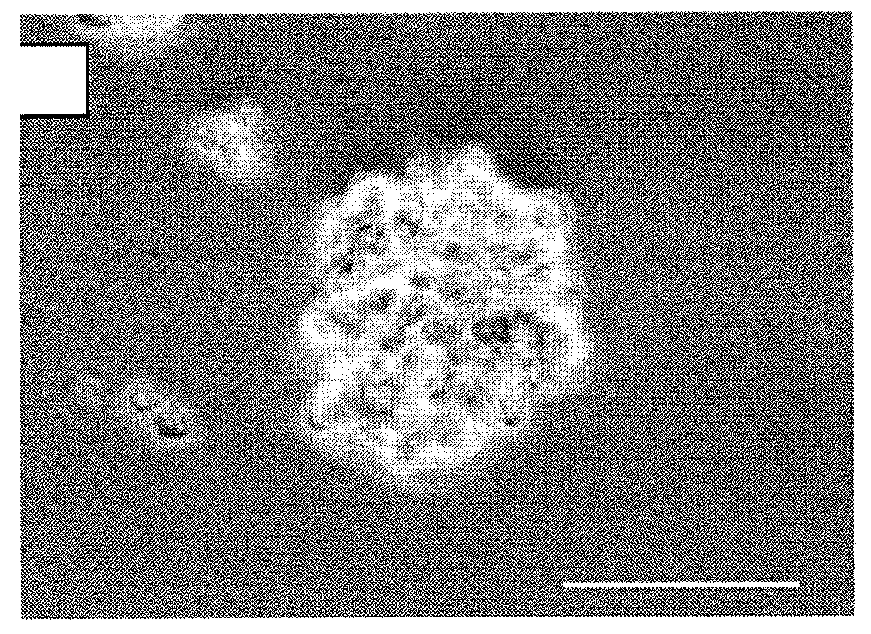
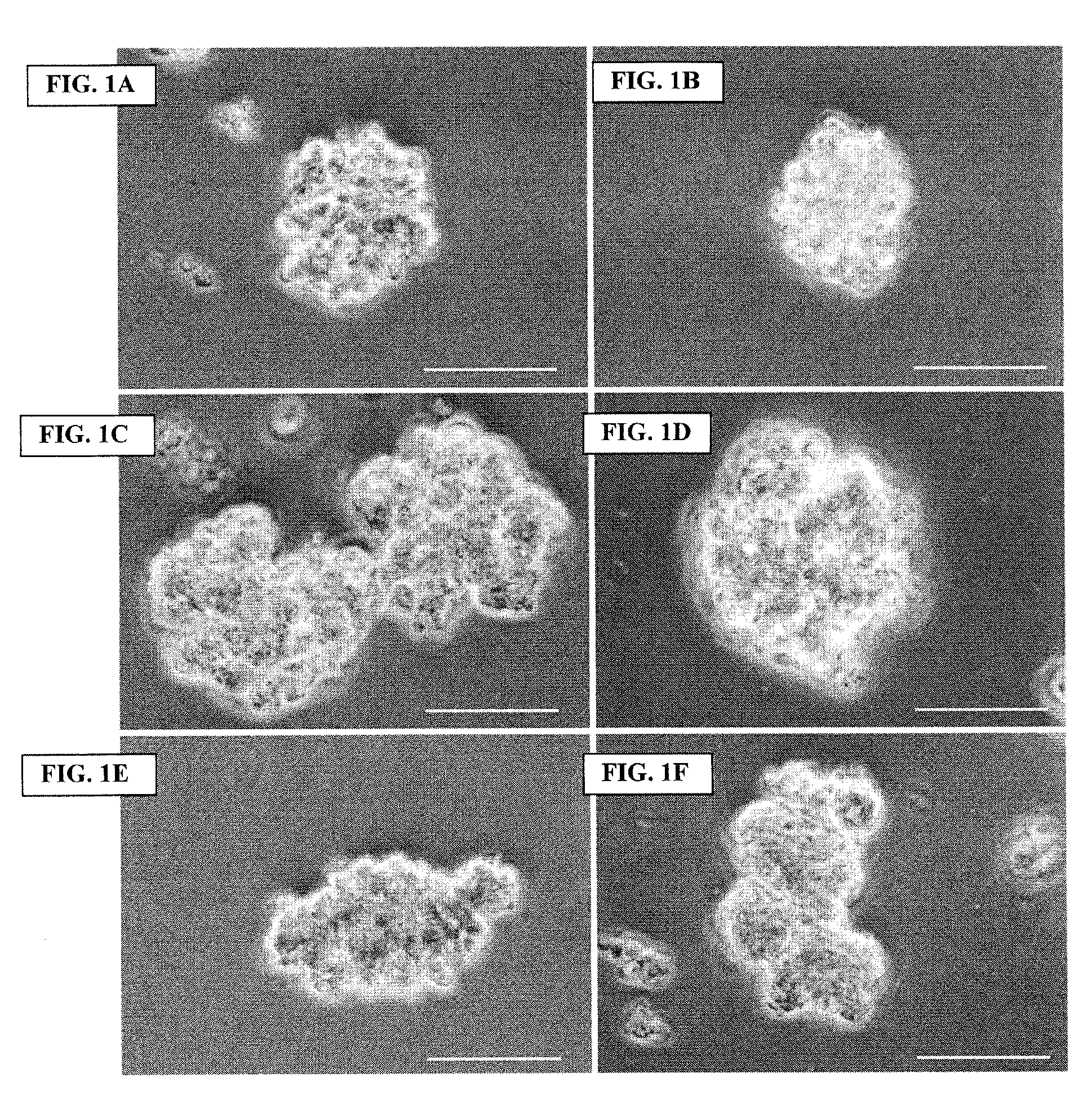
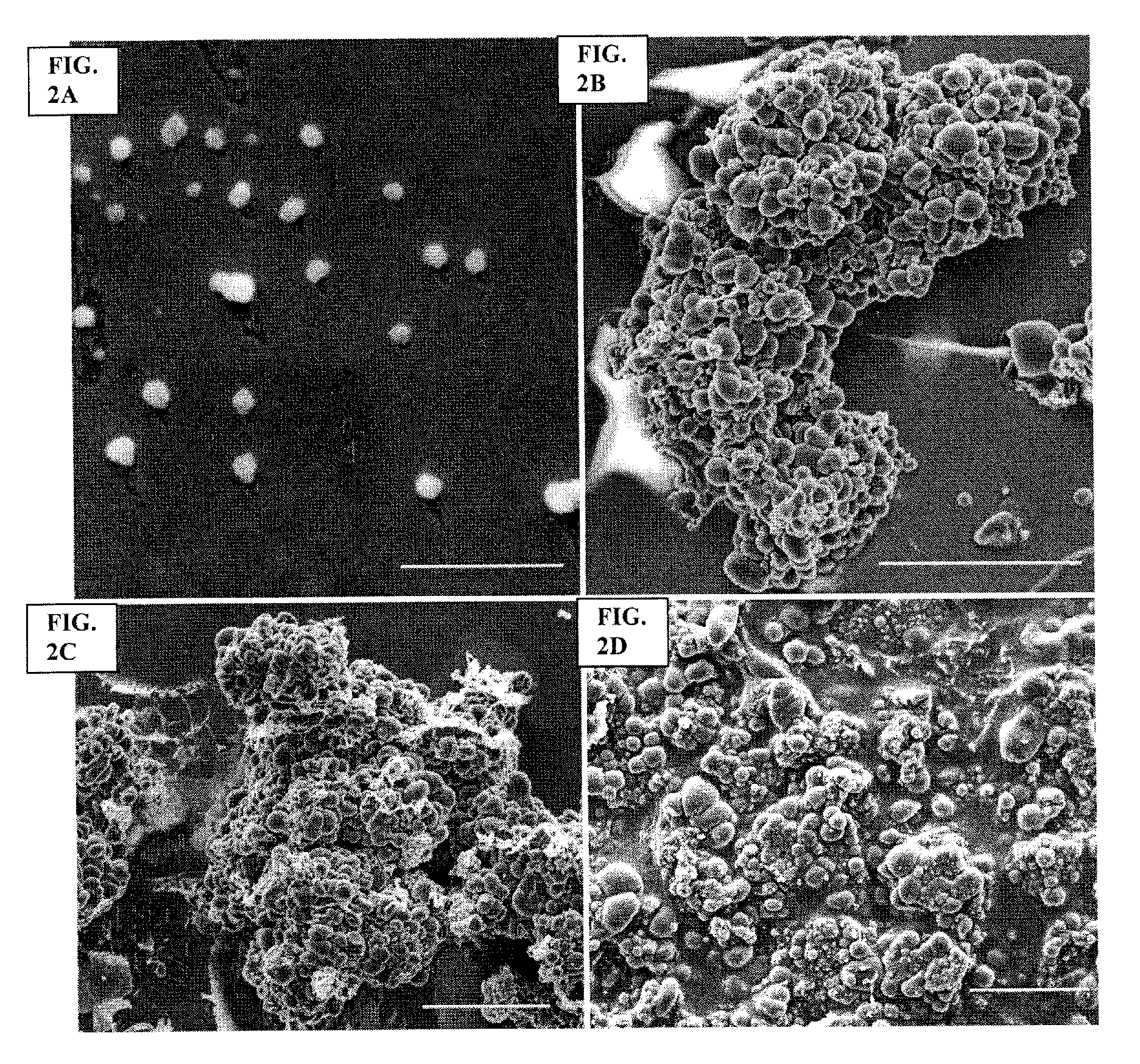
Archaeal polar lipid aggregates for administration to animals
a technology of lipid aggregates and lipid aggregates, which is applied in the direction of antibody medical ingredients, carrier-bound antigen/hapten ingredients, immunological disorders, etc., can solve the problem of little to no elicitation of mucosal immune responses, and achieve strong memory responses and memory responses. strong
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
2. The composition , wherein the multivalent cations are divalent cations.
3. The composition according to Embodiment 2, wherein the divalent cations are Ca2+.
4. A composition according to Embodiment 3, wherein the Ca2+ is provided as CaCl2.
embodiment 6
5. A composition , wherein in the total polar lipid extract from the archaeal species is mixed with the neutral lipids from the archaeal species.
6. A composition according to Embodiment 1, wherein the polar lipid extract is the total polar lipids extract from an archaeal species.
7. A composition according to Embodiments 1, 2, 3, 4, and 6, wherein the archaeal species is selected from the group consisting of Methanobrevibacter smithii, Halobacterium salinarum and Thermoplasma acidophilum.
8. A composition according to Embodiment 6, wherein the archaeal species is Methanobrevibacter smithii.
9. A composition according to Embodiment 6, wherein the archaeal species is Halobacterium salinarum.
10. A composition according to Embodiment 6, wherein the archaeal species is Thermoplasma acidophilum.
11. A composition according to Embodiment 1, wherein the polar lipid extract contains only one archaeal polar lipid.
embodiment 11
12. A composition , wherein the only one archaeal polar lipid in the extract is archaetidyl glycerophosphate-O-methyl.
13. A composition according to Embodiment 12, wherein the archaetidyl glycerophosphate-O-methyl is extracted from Halobacterium salinarum.
14. A composition according to Embodiment 1, comprising the following steps of preparation:[0141]a) preparation of unilamellar archaeosomes from a polar lipid extract from an archaeal species, with encapsulated pharmaceutical agent, biologically relevant molecule, or an antigen component;[0142]b) addition of CaCl2 to the archaeosome suspension, without prior removal of the un-encapsulated component, in sufficient quantity in excess to the proportion of the lipid, to form typical AMVAD structures characterized by aggregates of larger spherical structures possessing aqueous capture volume;[0143]c) removal of the free, soluble pharmaceutical agent, biologically relevant molecule, or antigen component from the preparation, and re-susp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com