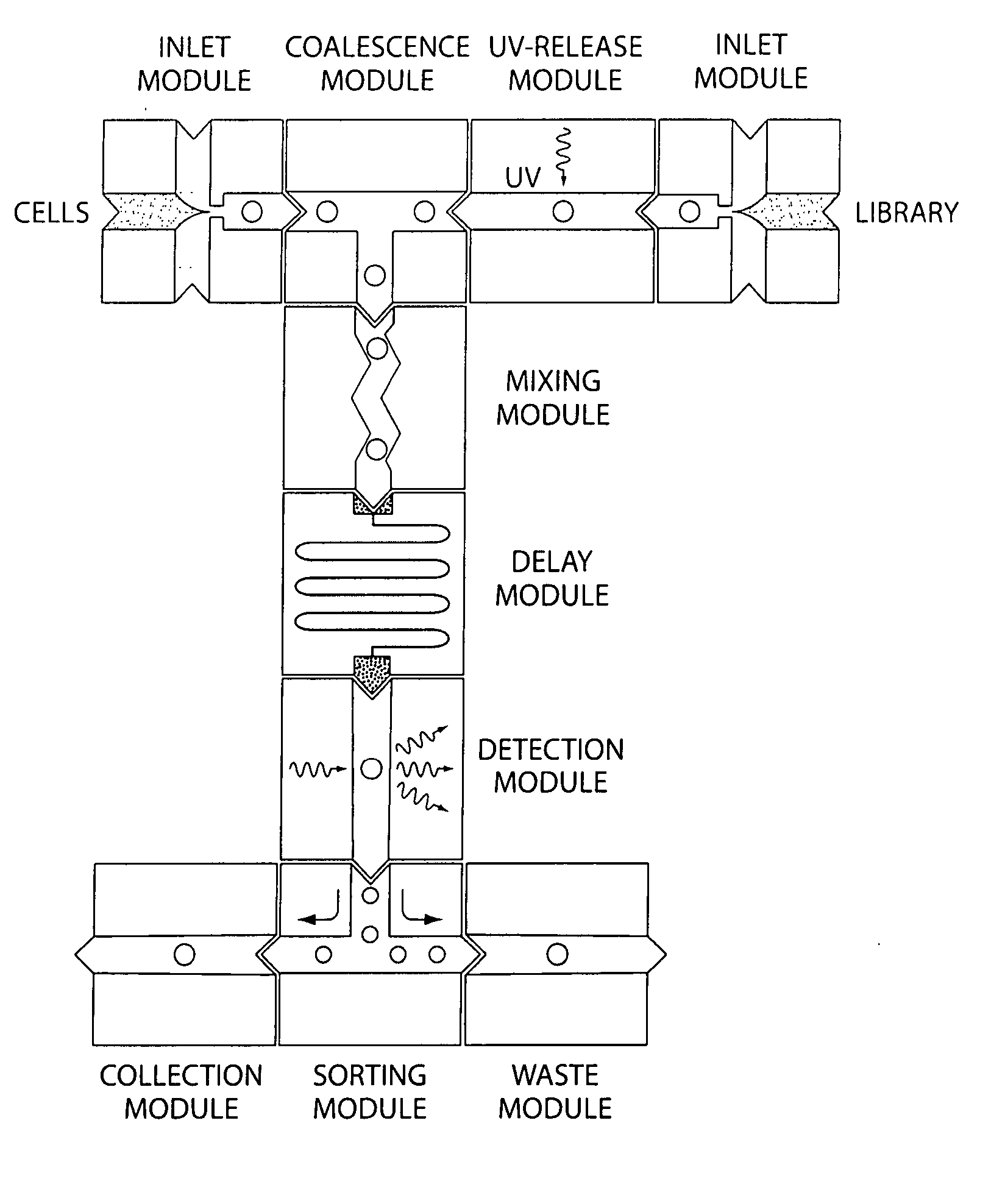
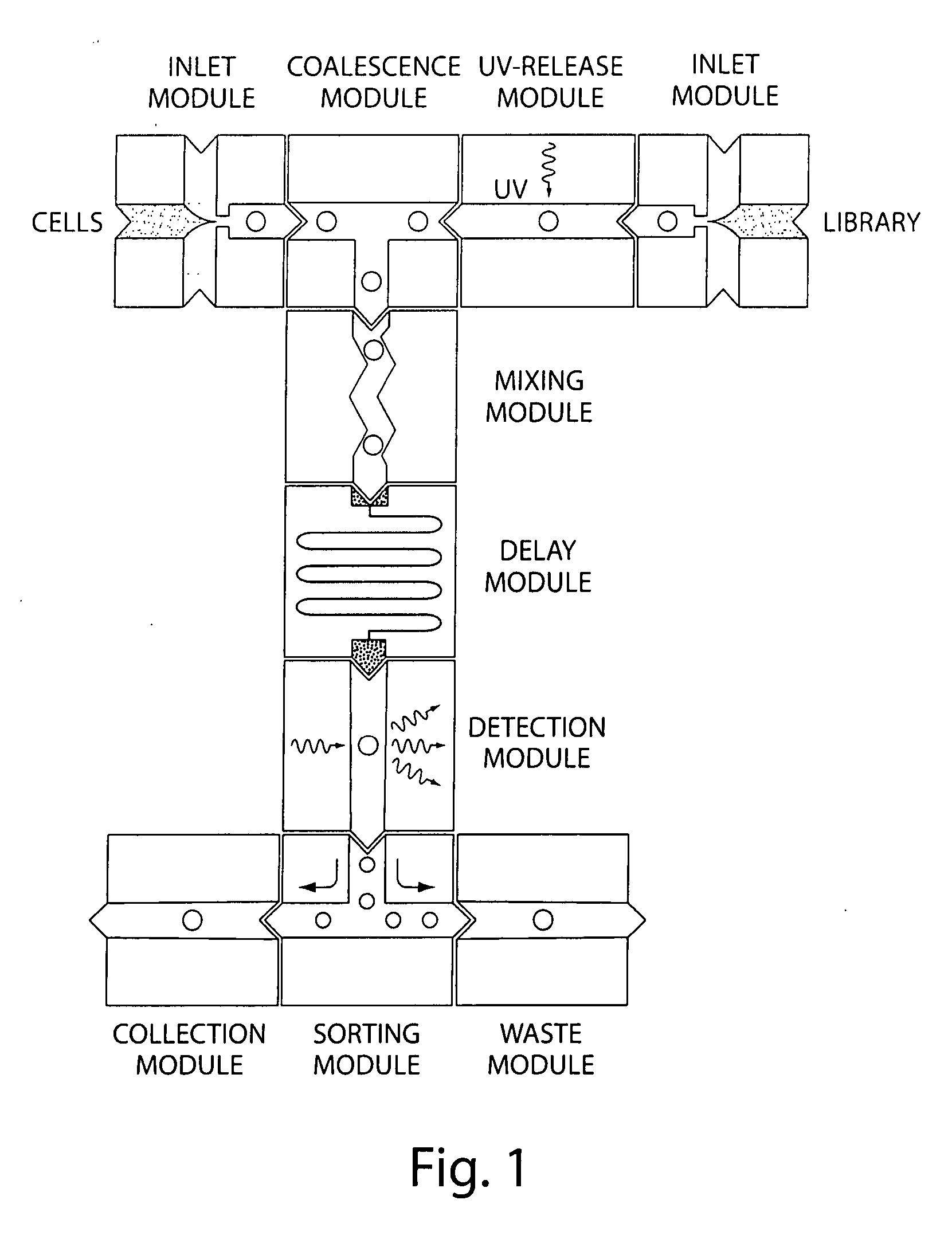
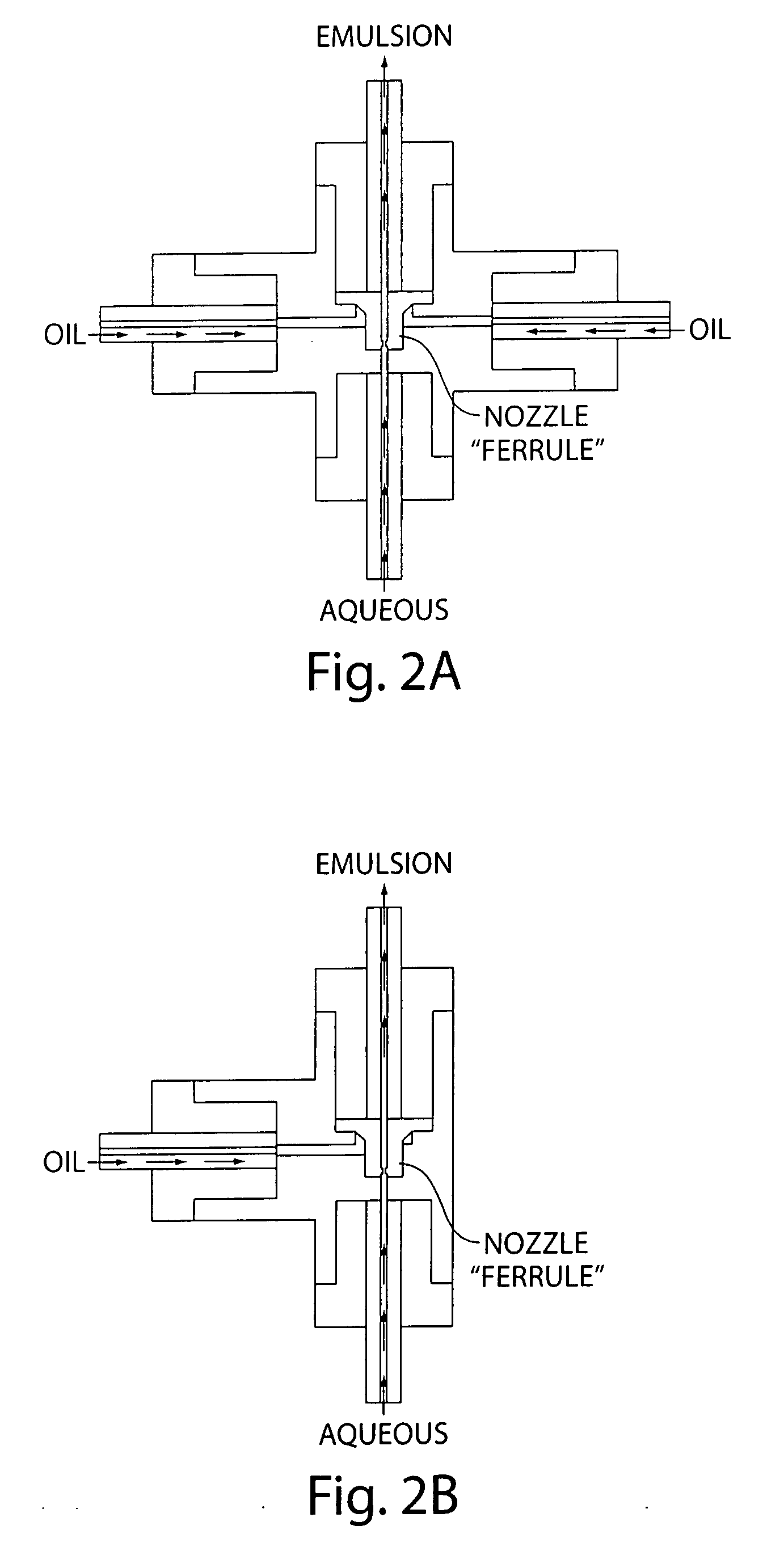
Microfluidic devices
a microfluidic device and microfluidic technology, applied in fluorescence/phosphorescence, laboratory glassware, instruments, etc., can solve the problems of high cost, high cost, and the limit of the smallest volume of reagent that can effectively be used, and achieve the effect of quick, effective and inexpensive us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0322] The present invention provides methods for preparing a library of droplet emulsions, where each of the droplets is of the same, predetermined size (monodisperse). Further, present invention provides methods for deterministic lateral displacement for continuous particle separation, which can occur within droplets on a microfluidic device.
[0323] Particles in solution are usually separated according to size by exclusion or hydrodynamic chromatography. In the former, a sample mixture is injected at one end of a tube packed with porous beads and then washed through the tube. Particles smaller than the pore sizes enter the beads, which lengthen their migration path, and so they are on average eluted later than larger particles. Zones of particles of a given size broaden, however, because particles in each zone take many different paths, leading to different retention times. This multipath effect reduces the resolution of size-exclusion chromatography. In hydrodynamic chromatograph...
example 2
[0334] The present invention provides methods for performing polymerase chain reaction (PCR). PCR can be performed on a drop-by-drop basis in a microfluidic device according to the present invention. A monolithic chip can be provided wherein the heating and cooling lines are built into the chip and a sorting means is provided. Advantages of performing PCR in droplets on such a chip are that the chip is disposable and the reaction can be repeated without cleaning the device between reactions. Furthermore, the chip provides a convenient way of getting all the components to perform PCR in the droplets in the right concentration. Additionally, the PCR is more efficient because the heat transfer is more efficient due to the small volume. This provides for shorter incubation / residence times. Droplets containing the nucleic acids, all PCR primers, and, if present, beads are generated one at a time at rates between 100 and 20,000 droplets per second. The droplets can then be sent through a ...
example 3
[0357] The present invention provides methods for performing isothermal-type amplification methods on a microfluidic device. Isothermal amplification is an alternative to the standard PCR techniques described herein. Isothermal amplification is used to reduce the relative amount of background DNA in a sample. Primers are generally used in a constant temperature means of amplification. Isothermal amplification is applicable for SNP detection. Once the DNA is amplified by isothermal amplification there are several well-known means for detecting which nucleotide polymorphism is present. These include, but are not limited to; allele specific primer extension, oligonucleotide ligation assay, mini-sequencing, fluorescence polarization, etc. Isothermal amplification is also applicable for DNA sequencing preparation. The isothermally-amplified DNA can be attached to a solid phase within a droplet or placed within a parking space on chip. The beads or parking spaces can be accessed and the a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com