Methods for sensing retrograde flows of bone fill material
a technology of bone filling and retrograde flow, which is applied in the field of medical devices for osteoplasty treatment procedures, can solve the problems of fractures in the spine and hips, affecting mobility and quality of life, and the advances in bone filling technology aimed at slowing or arresting bone loss from aging have not provided solutions to this problem, so as to prevent the migration of monomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Definitions
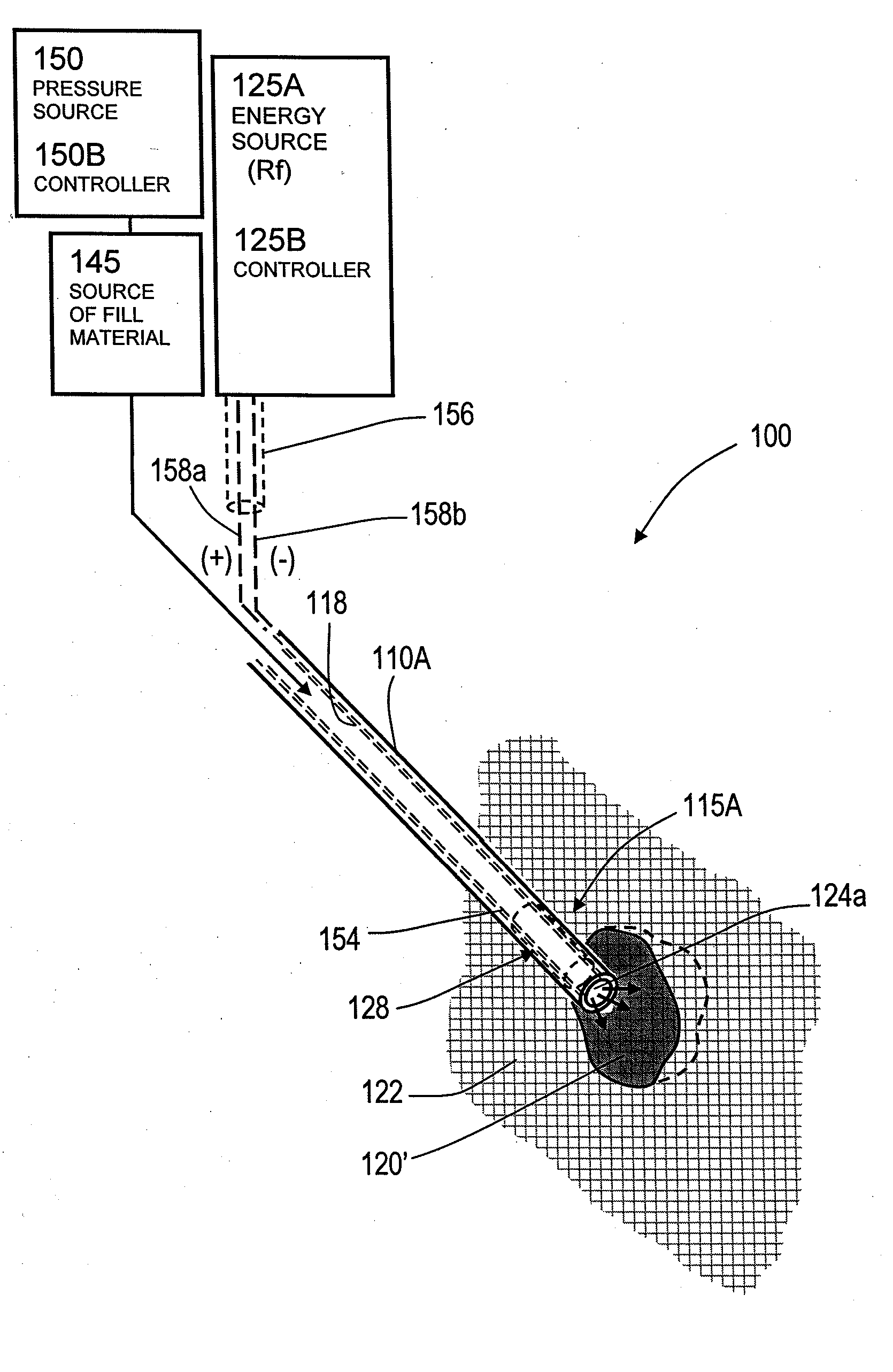
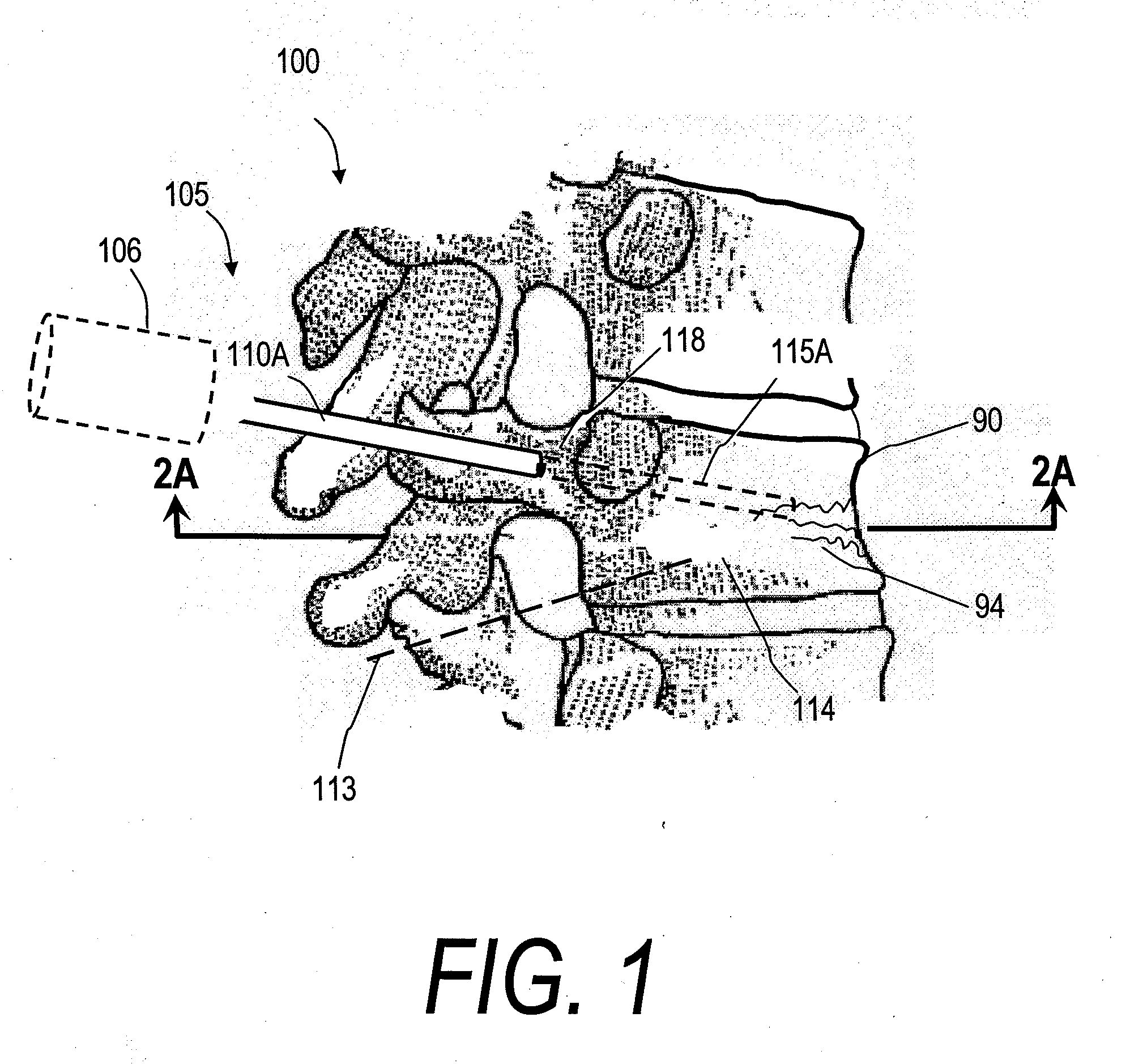
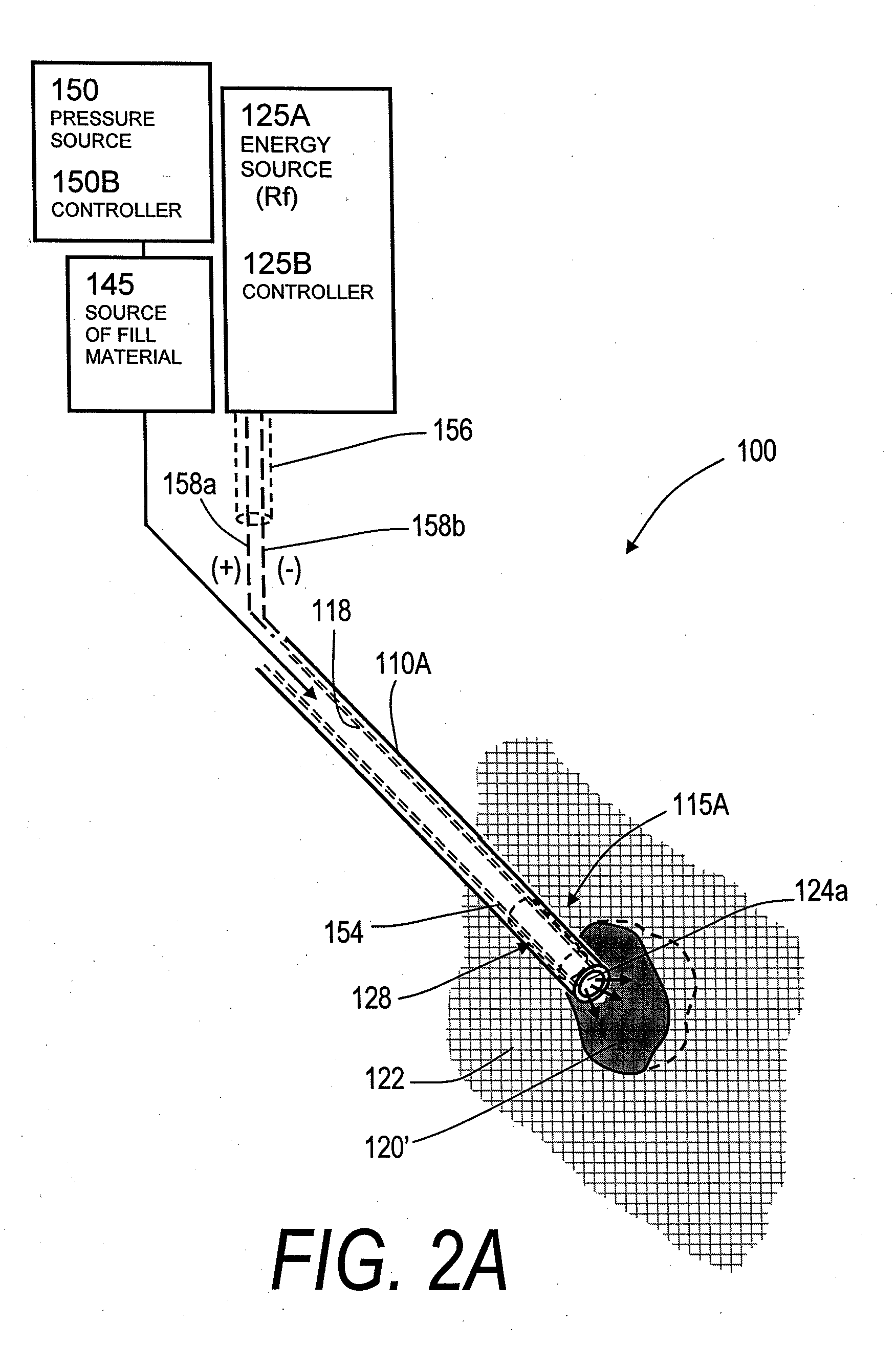
[0069]“Bone fill material, infill material or composition” includes its ordinary meaning and is defined as any material for infilling a bone that includes an in-situ hardenable material, such as bone cement. The fill material also can include other “fillers” such as filaments, microspheres, powders, granular elements, flakes, chips, tubules and the like, autograft or allograft materials, as well as other chemicals, pharmacological agents or other bioactive agents.
[0070]“Flowable material” includes its ordinary meaning and is defined as a material continuum that is unable to withstand a static shear stress and responds with an irrecoverable flow (a fluid)—unlike an elastic material or elastomer that responds to shear stress with a recoverable deformation. Flowable material includes fill material or composites that include a fluid (first) component and an elastic or inelastic material (second) component that responds to stress with a flow, no matter the proportions of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com