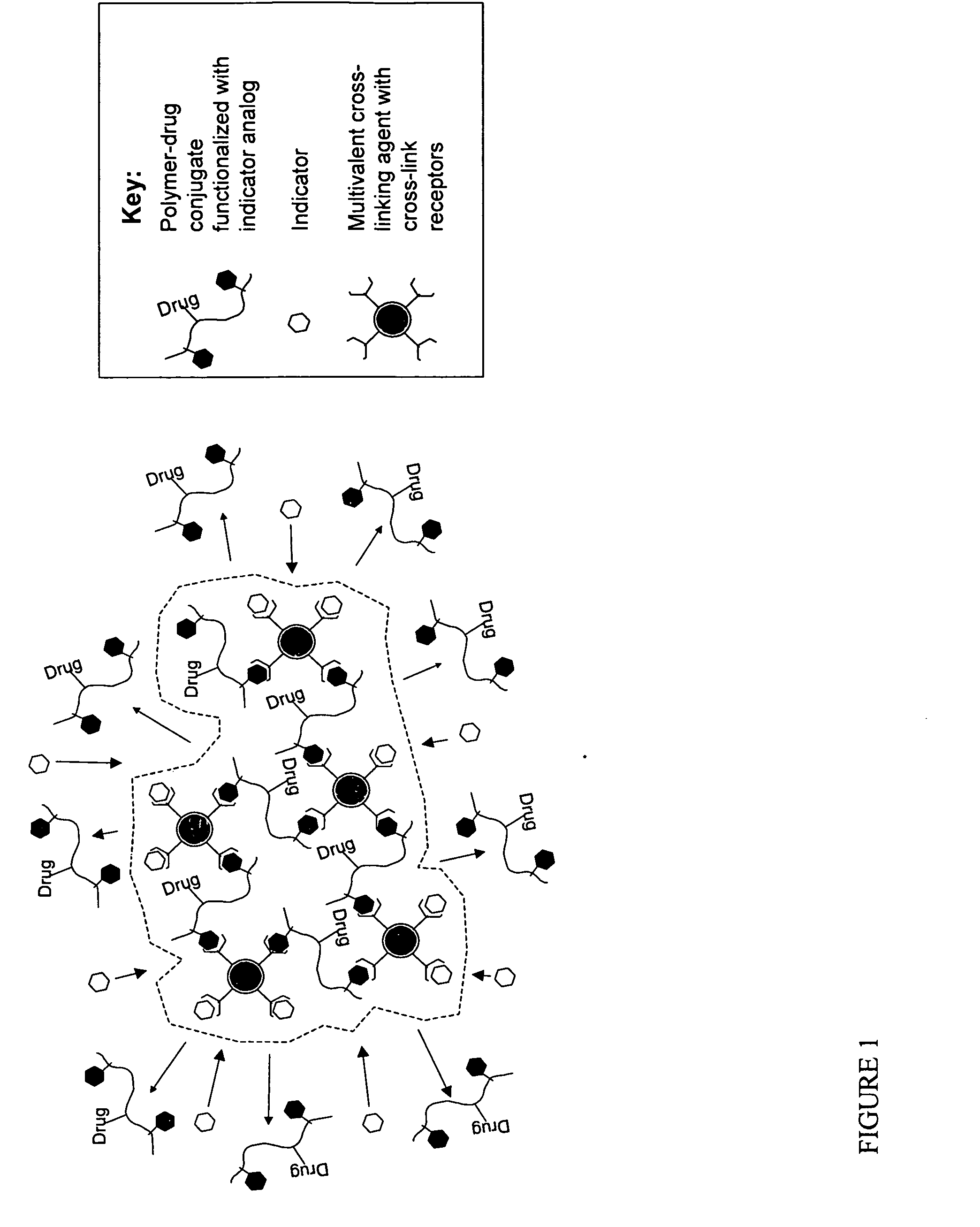
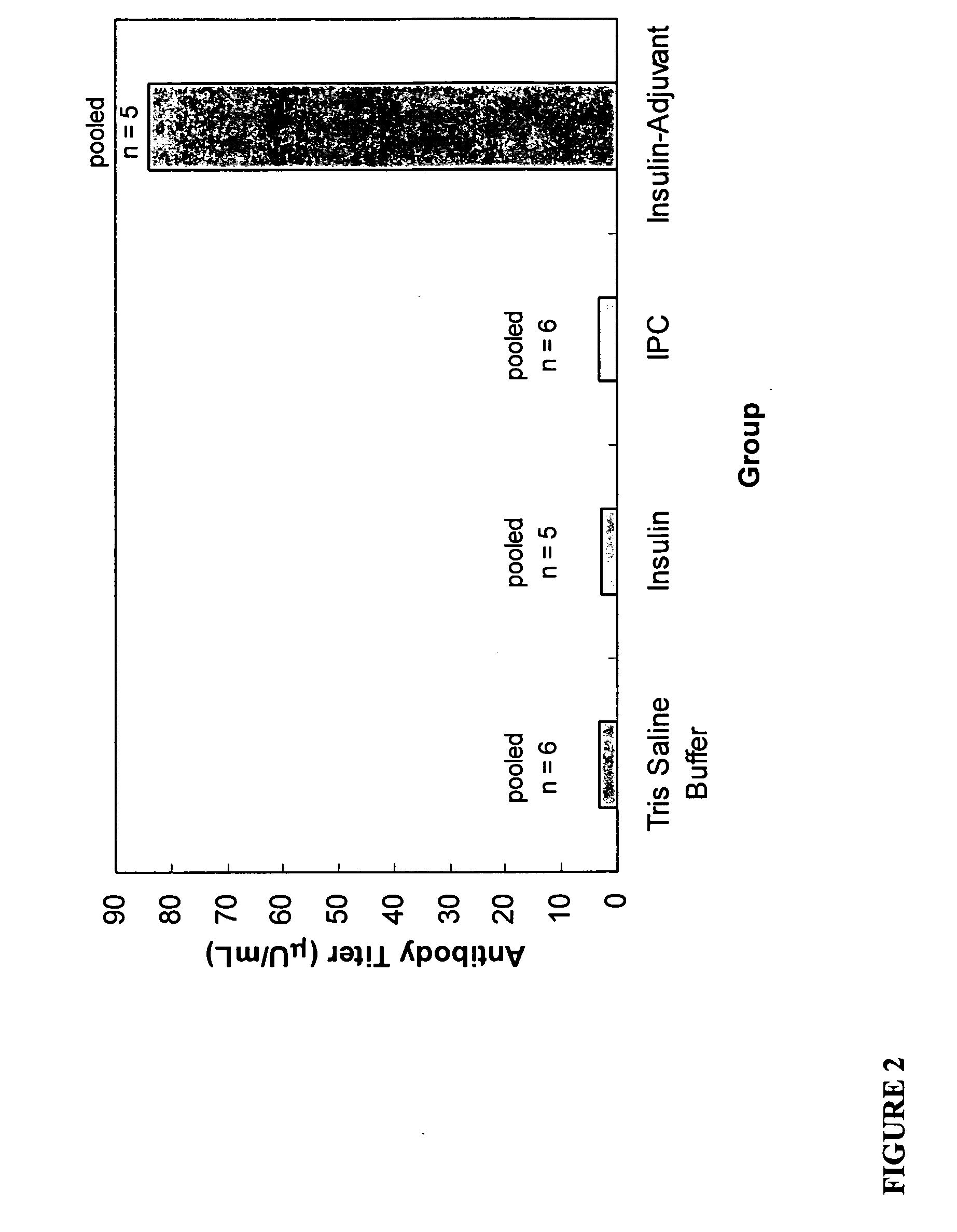
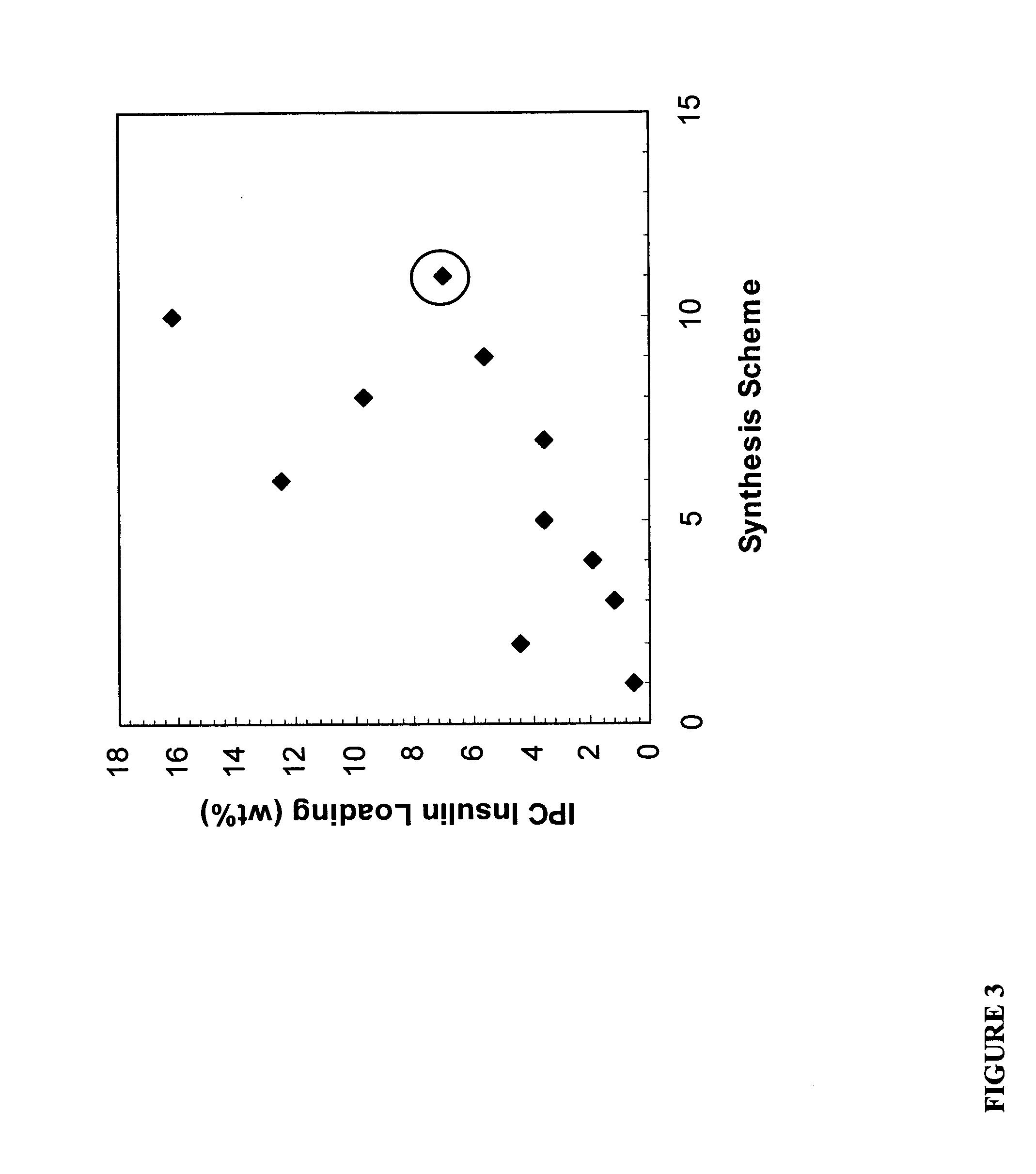
Polymer-drug conjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0087] Human recombinant insulin (HRI) is a well established and well characterized biologic protein made from E. coli. HRI is readily available in large, pharmaceutical-grade quantities from a number of manufacturers, including Eli Lilly, Novo Nordisk, and Diosynth. Insulin activity can be determined using an in vitro insulin receptor binding assay (e.g., see Example 13) and in vivo pharmacokinetic and pharmacodynamic studies using SD rat models (e.g., see later Examples).
[0088] Glycogen can be produced from Golden Bantam sweet corn (Curry Seed Company, Elk Point, S.Dak.) according to a modified procedure (Morris and Morris, J. Biol. Chem. 130:535-544, 1939). Briefly, 10 g of moist sweet corn kernels are ground and extracted with 3×100 ml of deionized water. The combined extracts are strained to remove coarse particles, and filtered through a fritted funnel to remove insoluble starches. The extract is then concentrated to one third the volu...
example 2
Generalized CNBr Conjugation Method
[0089] This example describes a generalized method for making insulin-glycogen conjugates using cyanogen bromide (CNBr) as a coupling agent. Briefly, a known mass of glycogen is dissolved in deionized water at a concentration of 10 mg / ml. Solid CNBr is added to the resulting solution at a CNBr to glycogen mass ratio between 0.05 and 1.5 and the pH maintained constant at 10.7+ / −0.2 using 3N sodium hydroxide (NaOH) solution. After stirring for 15 minutes, another equal mass of solid CNBr equal is added and the pH maintained constant at 10.7+ / −0.2 while stirring for 45 minutes. Insulin is then added to the solution at an insulin to glycogen mass ratio between 0.05 and 0.60 and the pH adjusted to 9.15 using solid sodium bicarbonate. The solution is stirred overnight, ultrafiltered exhaustively against deionized water using a 50 kDa MWCO polyethersulfone disc membrane filter (Millipore, Bedford, Mass.), and lyophilized. The resulting powder is then pu...
example 3
Generalized CDAP Conjugation Method
[0090] This example describes a generalized method for making insulin-glycogen conjugates using cyanodimethylamino-pyridinium tetrafluoroborate (CDAP) as a coupling agent. This synthesis was used to prepare various insulin-glycogen conjugates that are described in later Examples. The inventors have found that this method produces increased insulin loading as compared to the CNBr method of Example 2. Briefly, 8.0 g of glycogen is dissolved in 160 ml of 25 mM HEPES, 0.15 M NaCl, pH 9.0. 2.4-12.0 mL of a 1.0M CDAP solution in DMSO is added dropwise to the glycogen solution at 0° C. After stirring for 1 minute, a volume equal to that of the CDAP solution consisting of 0.2M triethylamine (TEA) is added dropwise over one minute. At this time, the pH of the reaction solution is adjusted to 9.0 using 1.2N HCl. Then, 80-2000 ml of a 10 mg / ml insulin solution in a 20 mM HEPES (pH 9.0) solution is added over the next three minutes and the pH adjusted again ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com