Powder mix of potassium calcium citrate for the treatment of kidney stones and osteoporosis
a potassium calcium citrate and kidney stone technology, applied in the direction of biocide, drug composition, sexual disorder, etc., can solve the problem of low risk of stone formation that might rise from calcium supplementation, and achieve the effect of chronic diarrheal syndrom
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
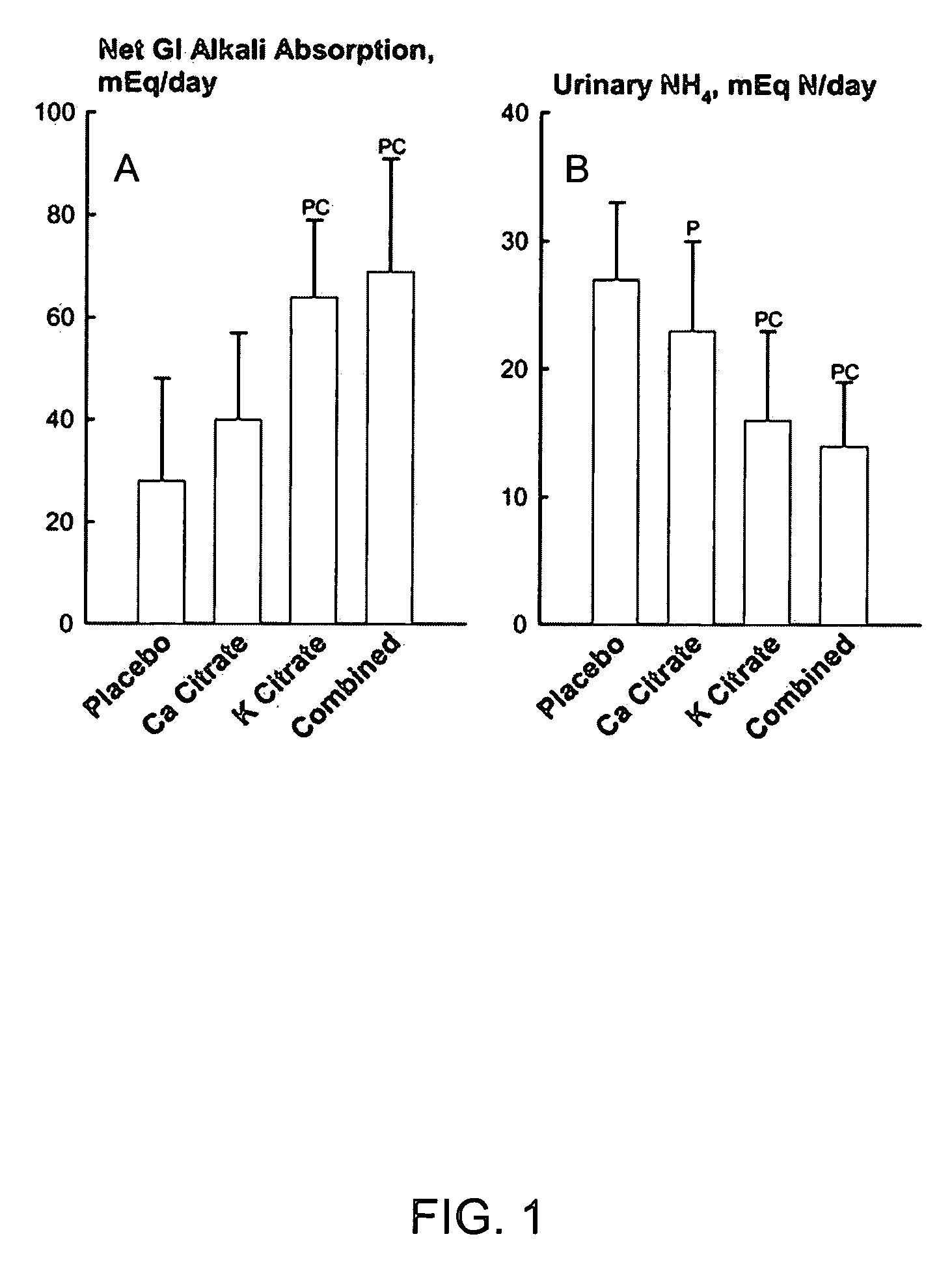
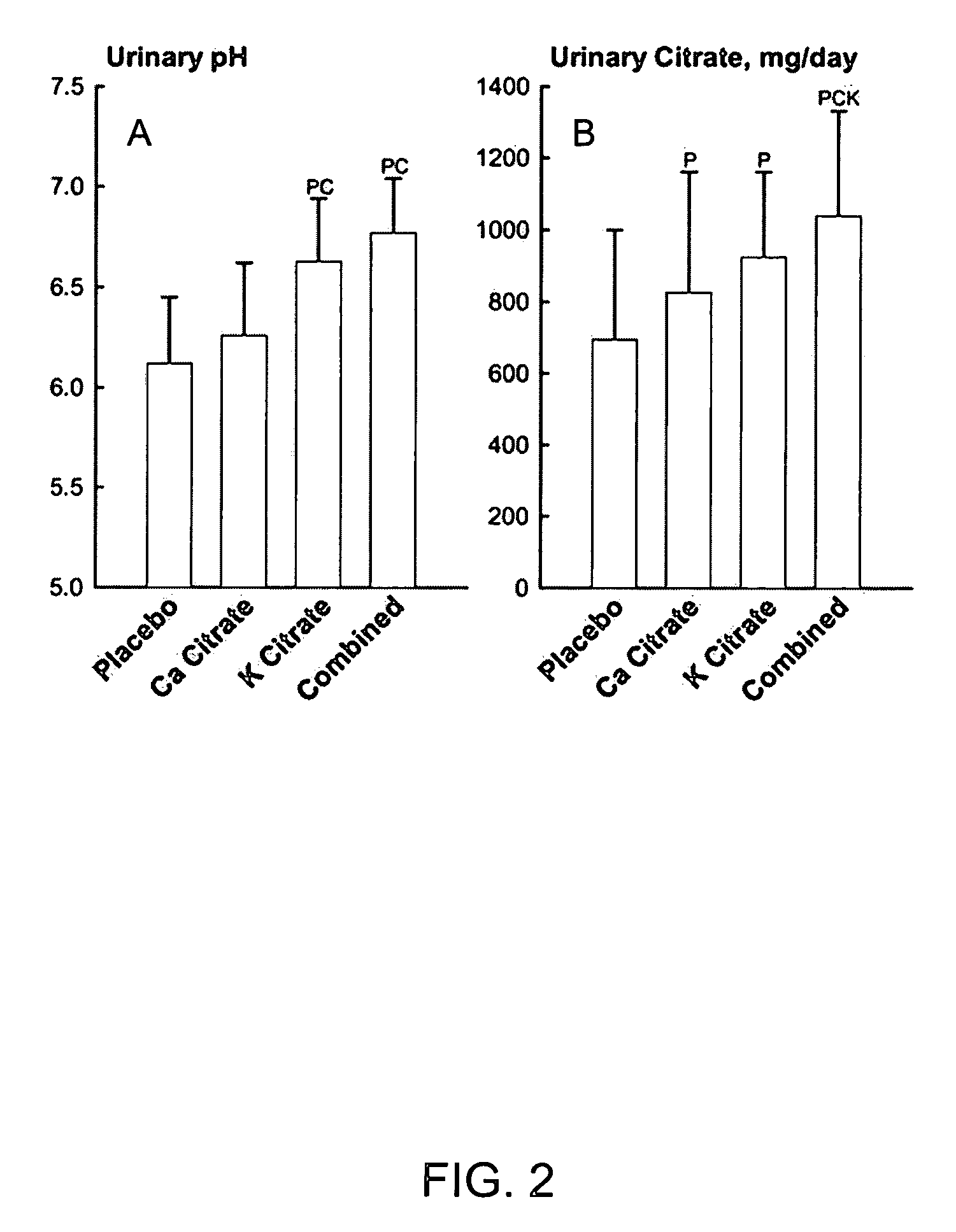
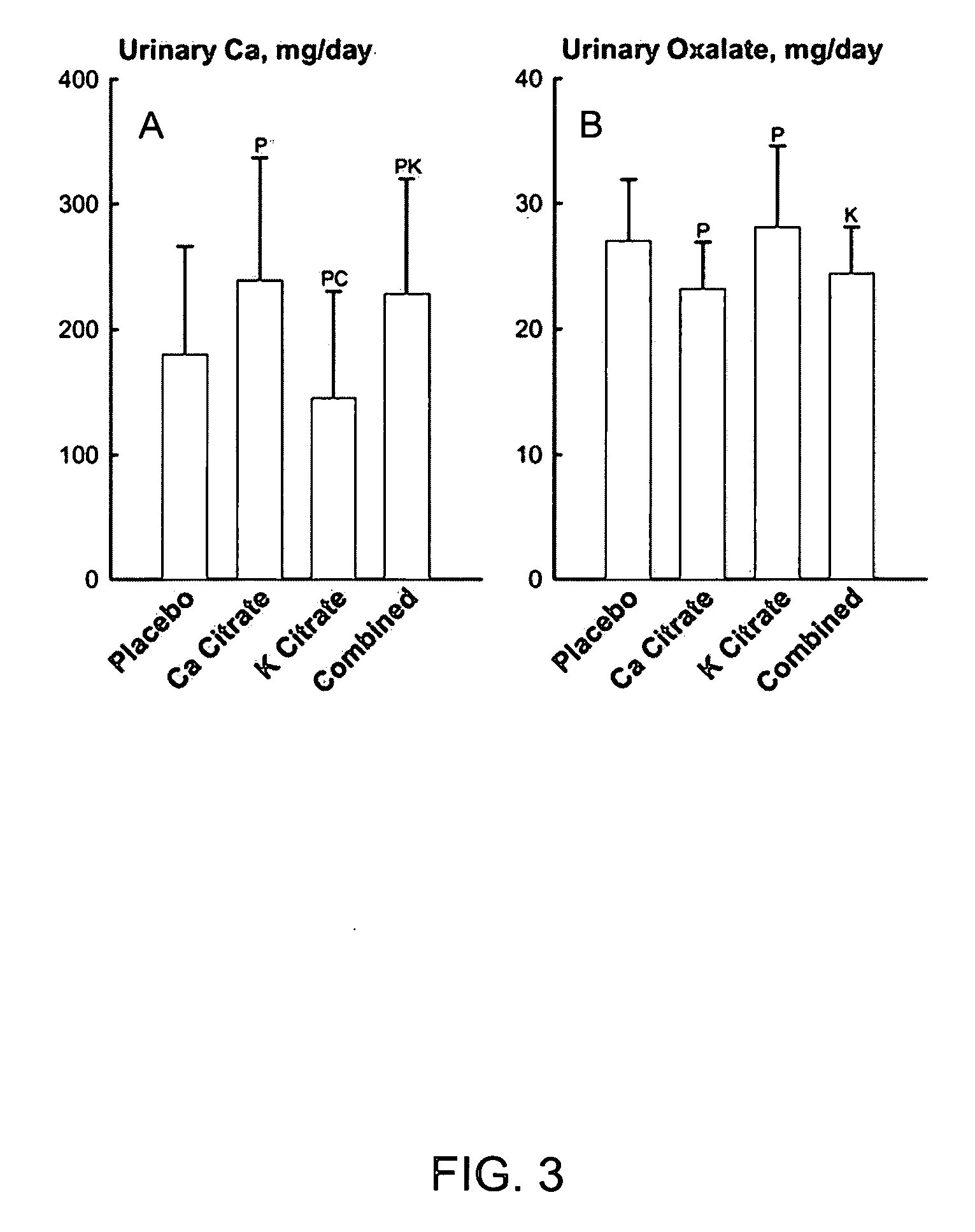
[0034] Metabolic Studies on Prevention of Kidney Stone Formation
[0035] A total of eighteen postmenopausal women without kidney stones underwent four phases of study chosen in random order while they were kept on a constant diet in a metabolic ward. Lasting two weeks each, the four phases comprised: potassium citrate (Urocit-K®) 20 mEq twice daily, calcium citrate (Citracal®) 400 mg calcium twice daily, both potassium citrate and calcium citrate at same dosages, and placebo. During last two days of each phase, urine was collected in 24-hour pools for biochemistry and stone risk factors. The combined treatment with potassium citrate and calcium citrate from this metabolic study is equivalent to treatment with potassium calcium citrate as embodied by this invention, since the amount of calcium and potassium conferred by combined treatment was about the same as the intended dose of the object of this invention.
[0036] Provision of Alkali Load.
[0037] The ability of calcium citrate and ...
example 2
[0045] Metabolic Studies on Preventing Bone Loss
[0046] In the same study involving the identical subjects described in Example 1, the effect on bone metabolism of potassium citrate, calcium citrate, and combination of the two was examined. During the last two days of each two-week phase, serum and 24-hour urine samples were collected for assessment of calcium metabolism, alkali load, and bone turnover markers.
[0047] Serum Calcium and Parathyroid Hormone.
[0048] Serum calcium and parathyroid hormone did not change with potassium citrate treatment (FIG. 5). Serum calcium increased slightly, and serum parathyroid hormone significantly decreased with calcium citrate. The combined treatment (resembling potassium calcium citrate) significantly increased serum calcium and marginally decreased serum parathyroid hormone.
[0049] Markers of Bone Turnover.
[0050] The effect of treatment on bone resorption (destruction) was evaluated from urinary hydroxyproline, urinary N-telopeptide, and seru...
example 3
[0054] Conditions Amenable to Treatment with Potassium Calcium Citrate
[0055] High Meat Diet.
[0056] The consumption of a high animal protein diet such as the Atkins' diet commonly taken for weight control, may increase the risk of kidney stones and bone loss. In a carefully conducted study in subjects consuming a constant diet, the Atkins' diet was shown to produce a marked acid load, nearly double urinary calcium, and lower urinary pH (making urine more acid) and citrate (inhibitor of calcium stone formation) (Reddy et al., 2002, Amer. J. Kid. Dis., 40: 265-274). Thus, the urine became more supersaturated with respect to stone-forming salts, making the formation of uric acid and calcium oxalate stones more likely. At the same time, the calcium balance turned more negative, because the intestinal calcium absorption was not changed even though urinary calcium increased.
[0057] Chronic Diarrheal Syndrome.
[0058] From the Stone Clinic of the inventors (CYCP and KS), 71 patients with c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com