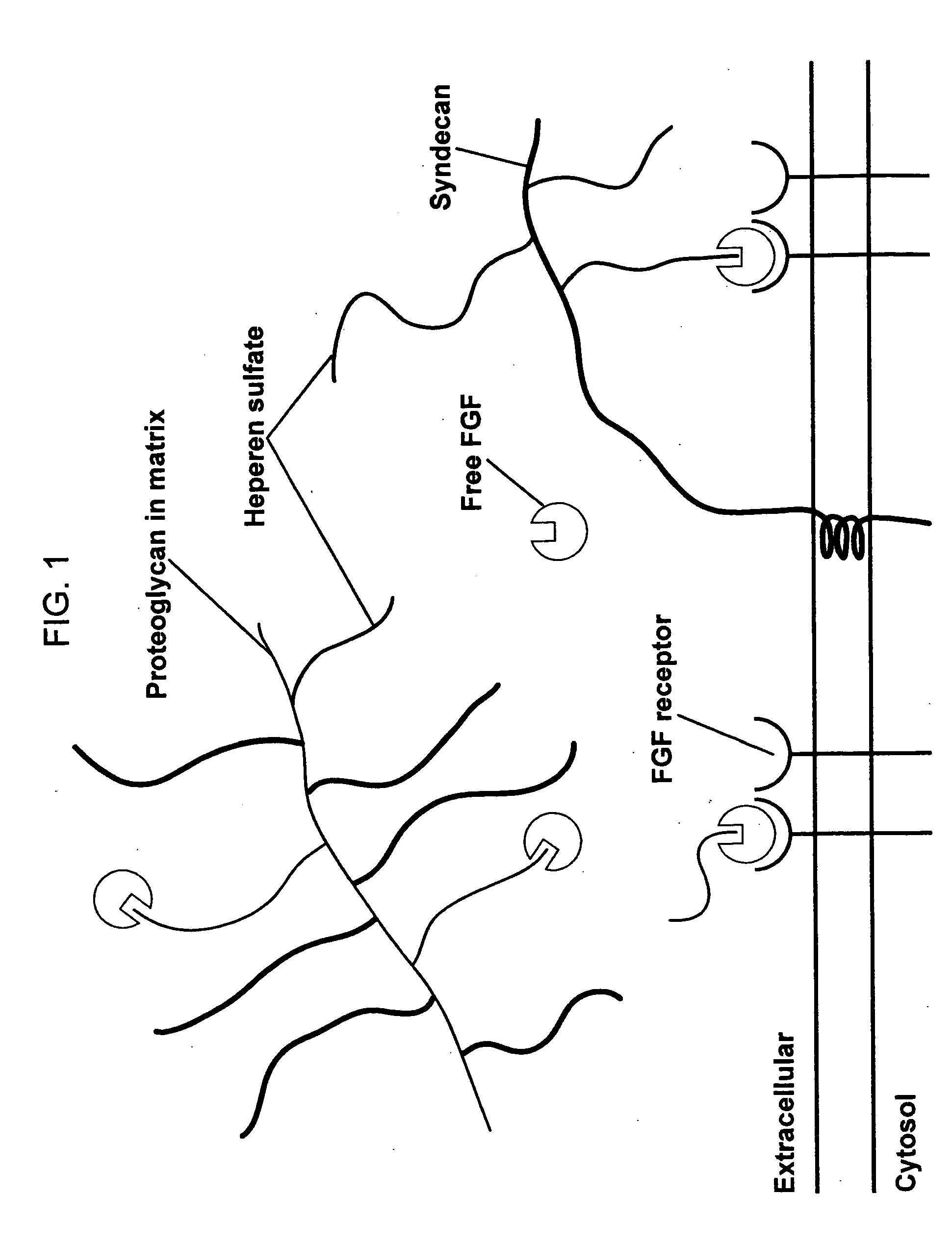
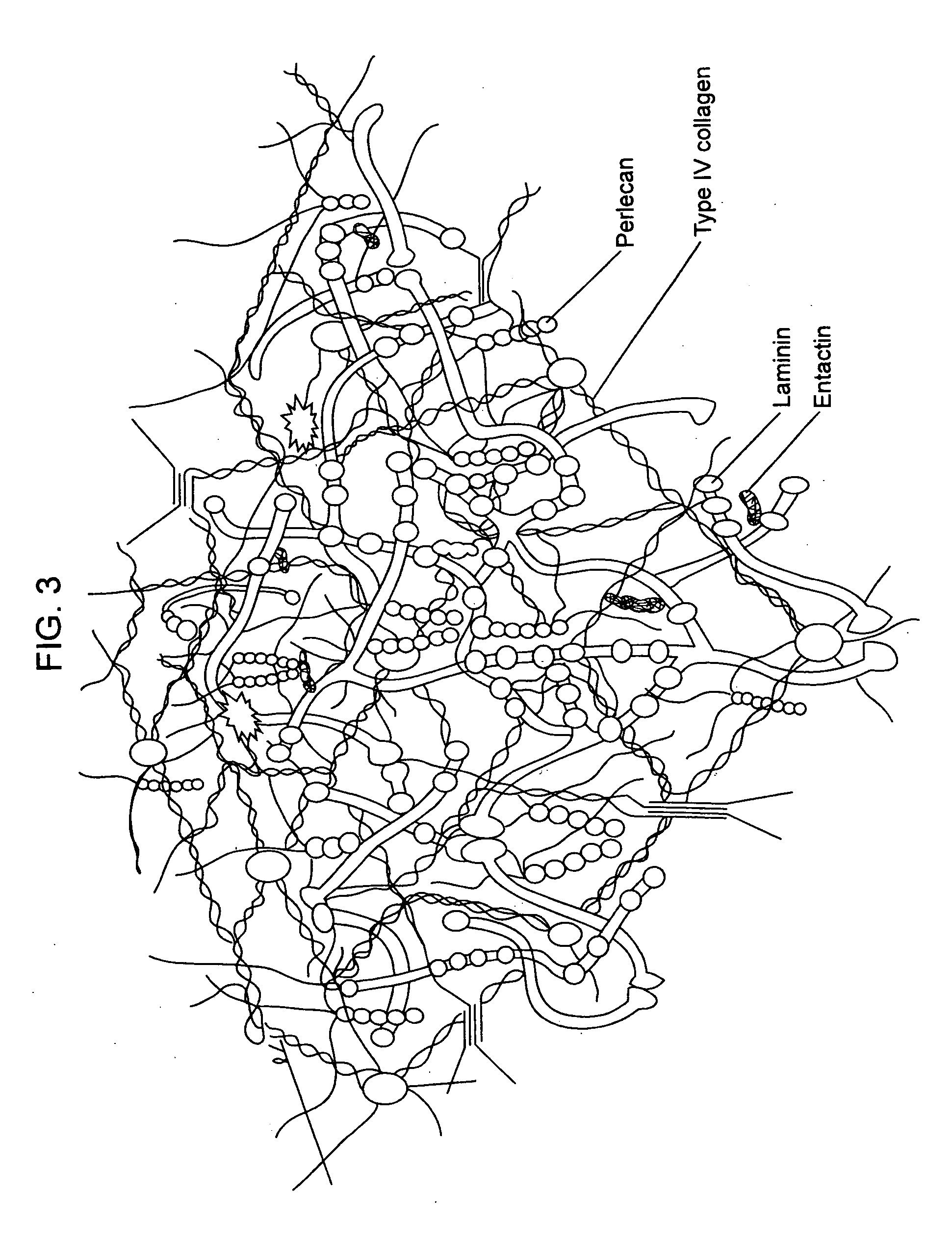
Compositions for regenerating defective or absent myocardium
a technology of myocardium and composition, applied in the field of tissue engineering, can solve the problems of high cost, high labor intensity, and high labor intensity, and achieve the effects of reducing labor intensity, reducing labor intensity, and reducing labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0100] An emulsion of urinary bladder submucosa (UBS) is prepared using standard emulsifying techniques. The emulsion is free of endogenous cells. This preparation is maintained as an emulsion by controlling the pH during storage of the emulsion before it is admininstered to the patient. In a minimally invasive procedure, a percutaneous catheter device is loaded with sufficient quantity of the emulsified UBS to address a defect in a human heart, the defect having been identified previously by imaging. The catheter is directed to the site of the myocardium in need of tissue regeneration using sonographic or radiographic imaging. Upon contact with the site, the emulsion is released and the catheter is withdrawn. The tissue regeneration process is monitored by sonography for several weeks or months post-delivery of the emulsion.
example 2
[0101] An emulsion of decellularized immunogenic liver basement membrane (LBM) is prepared using standard known techniques. While maintaining the emulsion state of the LBM, adult stem cells are co-cultured with the emulsion using standard stem cell culturing techniques. When the cells are ready, the entire composition is loaded into a catheter for percutaneous delivery to a human patient in need of tissue regeneration at a site of defective or absent myocardium. The emulsion with the co-cultured cells is delivered to the patient: a percutaneous catheter is loaded with the emulsion and directed to the site of the myocardium in need of tissue regeneration using sonographic or radiographic imaging. Upon contact with the site, the emulsion is released and the catheter is withdrawn. The tissue regeneration process is monitored by sonography for several weeks or months post-delivery of the emulsion.
example 3
[0102] An injectable emulsion of decellularized immunogenic stomach submucosa (SS) is prepared using standard known techniques. An aliquot of glycoaminoglycan (GAG) protein is covalently linked to some of the molecules of the matrix emulsion using standard covalent linking procedures for proteins. While maintaining the emulsive state of the SS, bone marrow progenitor cells are co-cultured with the emulsion using standard progenitor cell culturing techniques. An aliquot of transforming growth factor protein is added to the co-culturing composition before delivery to the human in need of myocardial tissue regeneration. The emulsion complete with cells and proteins is loaded into a percutaneous catheter which is directed to the site of the myocardium in need of tissue regeneration using sonographic or radiographic imaging. Upon contact with the site, the emulsion is released and the catheter is withdrawn. The tissue regeneration process is monitored by sonography for several weeks or m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com