Pharmaceutical for treatment of neurological and neuropsychiatric disorders
a neuropsychiatric and drug technology, applied in the direction of drug compositions, group 5/15 element organic compounds, animal repellents, etc., can solve the problem of increasing the likelihood of firing an action potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
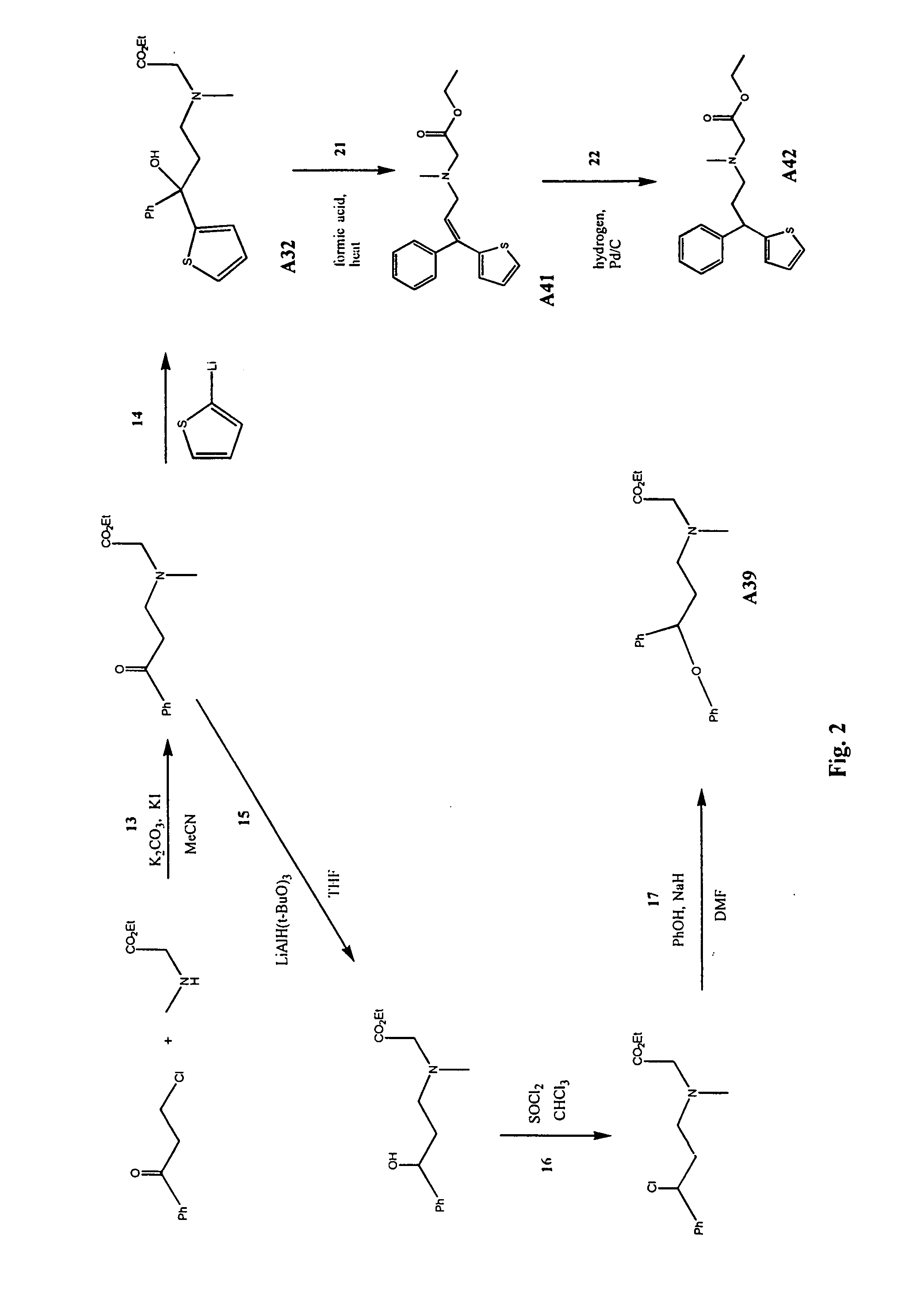
Synthesis of N-[(4,4-Diphenyl)but-3-enyl]glycine ethyl ester (Compound A26)
[0121] A mixture of 5.95 g (20.7 mmol) 4-bromo-1,1-phenyl-1-butene (prepared as described in F. A. Ali et al., J. Med. Chem., 28: 653-660, 1985), 4.71 g (33.7 mmol) glycine ethyl ester hydrochloride (Aldrich, Milwaukee, Wis.), 11.62 g (84 mmol) potassium carbonate and 1.06 g (6.38 mmol) potassium iodide in 50 ml acetonitrile was refluxed with stirring under argon for seven hours. The reaction mixture was filtered, the solvent evaporated and the residue chromatographed on silica gel column with 20% ethyl acetate in hexanes to give 3.70 g (yield 58%) of N-[(4,4-diphenyl)but-3-enyl]glycine ethyl ester (compound A26) as an oil. NMR spectra of the product showed: 1H NMR (CDCl3, 300 MHz) 7.60-7.00 (m, 10H), 6.09 (t, 1H), 4.16 (q, 2H), 3.35 (s, 2H), 2.71 (t, 2H), 2.32 (dt, 2H), 1.25 (t, 3H), 13C NMR (CDCl3, 75 MHz) 172.29, 143.25, 142.37, 139.82, 129.72, 128.13, 128.04, 127.97, 127.13, 126.92, 126.88, 126.68, 60.56...
example 2
Additional Syntheses According to Reaction 1
[0122] Additional compounds were synthesized using Reaction 1, as follows:
Amino acid orCompoundReagentprecusorSolventYieldA11BX27%A21CX35%A77EX9%A94EX47%A111AX70%A124EX7%A142DX15%A186EX50%A235EX26%A243DY20%A438FX12%A529FX28%A5710FX31%A6711FX10%A7112EX28%A7513FX73%A7714FX36%A8515FX86%A8716FX59%A9017EX16%A9517FX65%A9617EX50%A10415EX62%A10618FX65%A12119EX3%A12219EX40%A12319FX72%A13020EX6%A13221FX90%A13421EX67%A1706FX72%A4822Fx87%A5023FX81%A5324FX76%A5925FX77%A6126FX91%A6327FX91%A7028FX89%A7329FX86%A7430FX76%A7831FX49%A8032FX66%A8233FX38%A8333EX25%A8834FX55%A8935FX75%A9936FX56%A10037FX67%A11138FX34%A11739FX58%A11840FX89%A12041FX62%A12542FX46%A12643EX57%A12744EX5%A12844EX53%A12944FX66%A13845FX48%A14046FX69%A14147FX51%A14248FX67%A14349FX61%A14550FX98%A15551FX70%A15652FX65%A15853FX59%A15954FX85%A16055FX87%A17156FX88%A17357FX81%A17758FX84%A17858FX60%A17959FX68%A18024GX85%
1) 4-bromo-1,1-diphenyl-1-butene, (prepared as described in F. ...
example 3
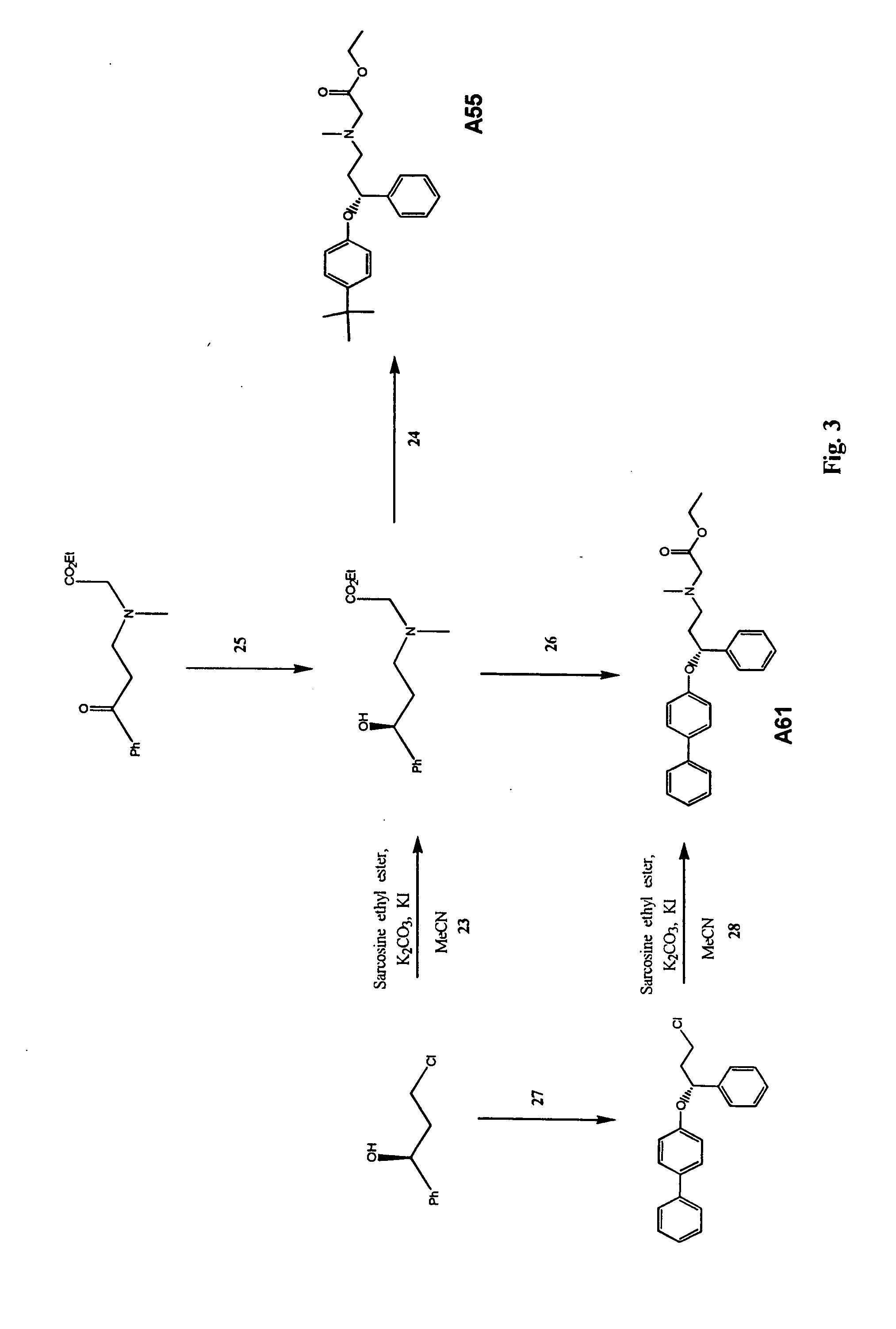
Synthesis of N-[(3,3-Diphenyl)propyl]glycine ethyl ester (Compound A22)
[0124] 2.132 g (10.1 mmol) 3,3-diphenylpropylamine (Aldrich, Milwaukee, Wis.) was added to a mixture of 0.853 g (5.11 mmol) ethyl bromoacetate (Aldrich) and 2.7 g (19.57 mmol) potassium carbonate in 14 ml acetonitrile at rom temperature. The mixture was stirred under argon for 18 hours. The reaction mixture was filtered, the solvent evaporated and the residue chromatographed on a silica gel column with 40% ethyl acetate in hexanes to give 1.05 g (yield 69%) N-[(3,3-diphenyl)propyl]glycine ethyl ester (Compound A22) as an oil. NMR spectra of the product showed: 1H NMR (CDCl3, 300 MHz) 7.40-7.10 (m, 10H), 4.14 (q, 2H), 4.03 (t, 1H), 3.33 (s, 2H), 2.56 (t, 2H), 2.24 (dt, 2H), 1.22 (t, 3H); 13C NMR (CDCl3, 75 MHz) 172.44, 144.66, 128.43, 127.75, 126.15, 60.63, 50.93, 48.80, 47.92, 35.85, 14.17, 0.019 g of A28 was also isolated from the silica gel column.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com