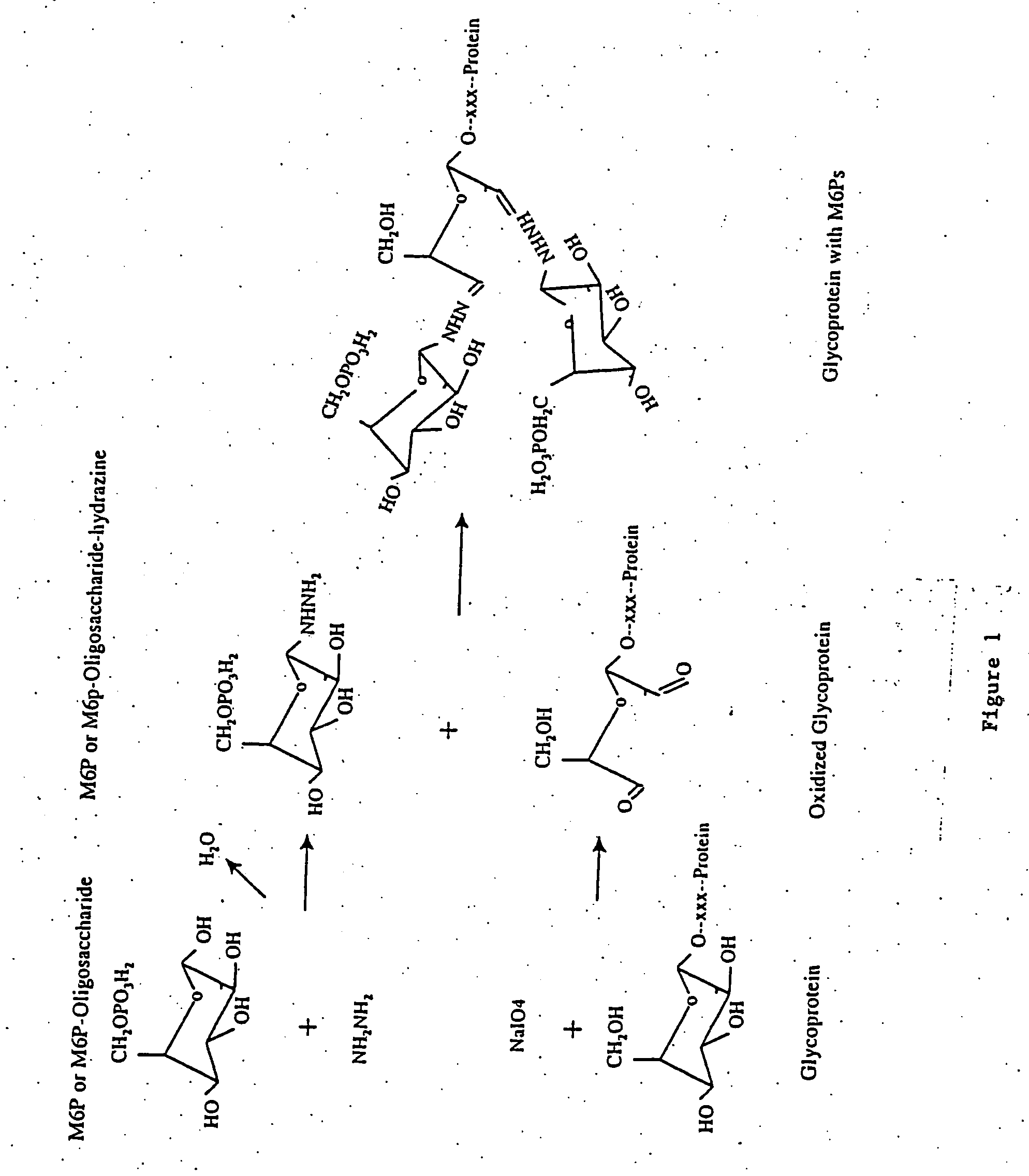
Methods for introducing mannose 6-phosphate and other oligosaccharides onto glycoproteins
a glycoprotein and oligosaccharide technology, applied in the direction of drug compositions, peptide sources, metabolic disorders, etc., can solve the problems of destroying biological activity, changing protein conformation, and destroying protein biological activity, so as to enhance the cellular uptake of such glycopropteins and enhance the uptake of enhanced glycoproteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Phosphopentamannose-Hydrazine Derivatives
[0068] Phosphopentamannose was prepared from phosphomanan obtained from Dr. M. E. Slodki, Northern Regional Research Laboratory, US department of agriculture, Peoria, Ill. phosphopentamannose was prepared essentially as described by M. E. Slodki (1962) and has the following structure: 6-P-M(alpha 1,3)-M(alpha 1,3)-M(alpha 1,3)-M(alpha 1,2)-M.
[0069] 100 mg of lyophilized powder of phosphopentamannose was added into a glass tube, to which 3 ml of anhydrous hydrazine was added. The tube was filled with nitrogen gas, capped with a tight fitting cap, and wrapped with parafilm. The reaction was proceeded at room temperature for 6-18 hours, after which the hydrazine was evaporated under vacuum while the hydrazine was absorbed through a bottle of sulfuric acid. 2 milliliters of toluene was added and removed by a stream of nitrogen gas to get rid of the residual hydrazine (Tolvanen and Gahmberg, 1986, supra; Gahmberg and Tolvanen, 1994,...
example 2
Coupling of Phosphopentamannose-Hydrazine to Avidin
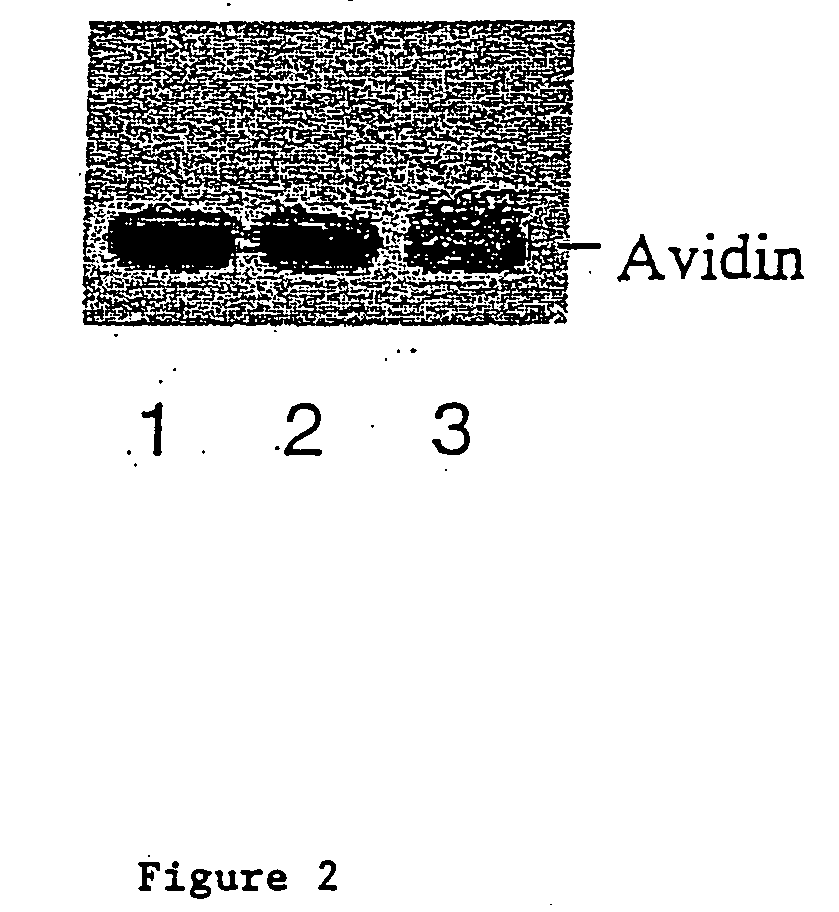
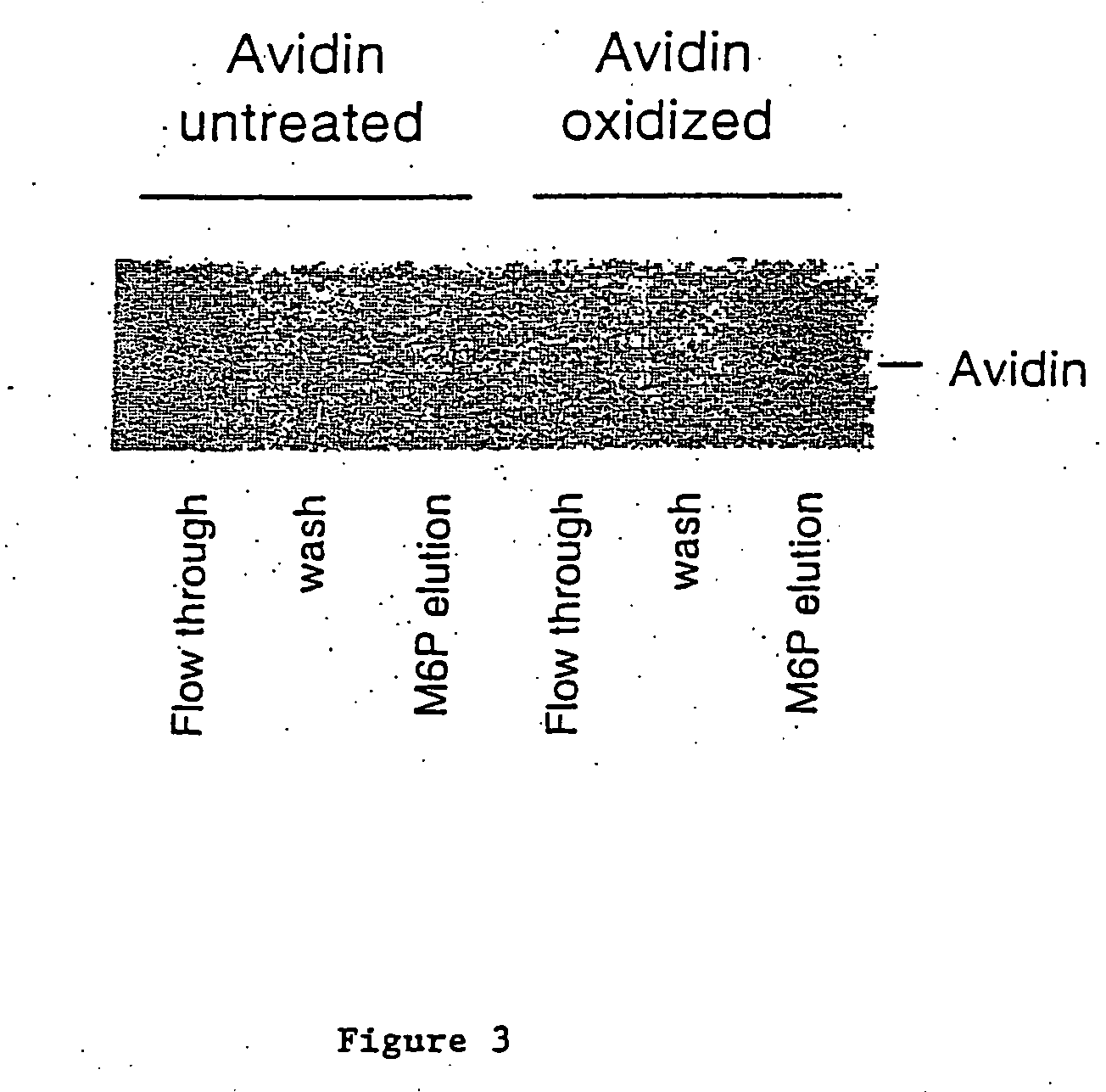
[0070] Oxidation of Avidin:
[0071] 1 ml of 2.5 mg / ml of avidin (obtained from Sigma or Pierce) were oxidized with 10 mM sodium periodate in 100 mM sodium acetate (pH 5.6) for 30 minutes at 4 degree centigrade in the dark. After which 25 microliters of glycerol were added and the sample was incubated on ice for 15 minutes to consume the excess sodium periodate. Samples were then dialyzed overnight against 100 mM sodium acetate (pH 5.6) at 4 degree centigrade. 0.5 ml of 2.5 mg / ml avidin without periodate oxidation were processed the same way as untreated control. Samples after dialysis were collected and stored at 4 or −20 degree centigrade until use.
[0072] Coupling of Posphopentamannose-Hydrazine to Oxidized Avidin:
[0073] 200 microliters of untreated or oxidized avidin (2.5 mg / mi) were mixed with 1 mg of phosphopentamannose-hydrazine dissolved in 20 microliters of 100 mM sodium acetate buffer (pH 5.6) and incubated at 37 degree ce...
example 3
Conjugation of Phosphopentamannose-Hydrazine to Beta-Glucuronidase
[0078] One major concern about the conjugation is that lysosomal enzymes conjugated in such a way must retain enzymatic activity, preferably full activity. While the avidin conjugation result clearly has shown that the coupling process is highly efficient, whether the coupling process affect its biological activity is unknown, in particular, avidin is a stable protein, not an enzyme. Therefore in the following example, lysosomal enzyme beta-glucuronidase isolated from bovine liver (50,000 U / mg, not completely pure, purchased from Sigma) was used.
Oxidation and Coupling Processes Do Not Inactivate Beta-Glucuronidase:
[0079] 6 mg of beta-glucuronidase were dissolved in 1.5 ml of water, 1.3 ml of the material (4 mg / mI) were dialyzed against 100 mM NaAc (pH 5.6) overnight at 4 degree centigrade. 200 microliters of the rest sample were kept at 4 degree centigrade as water-control.
Oxidation:
[0080] Of the sodium acetate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com