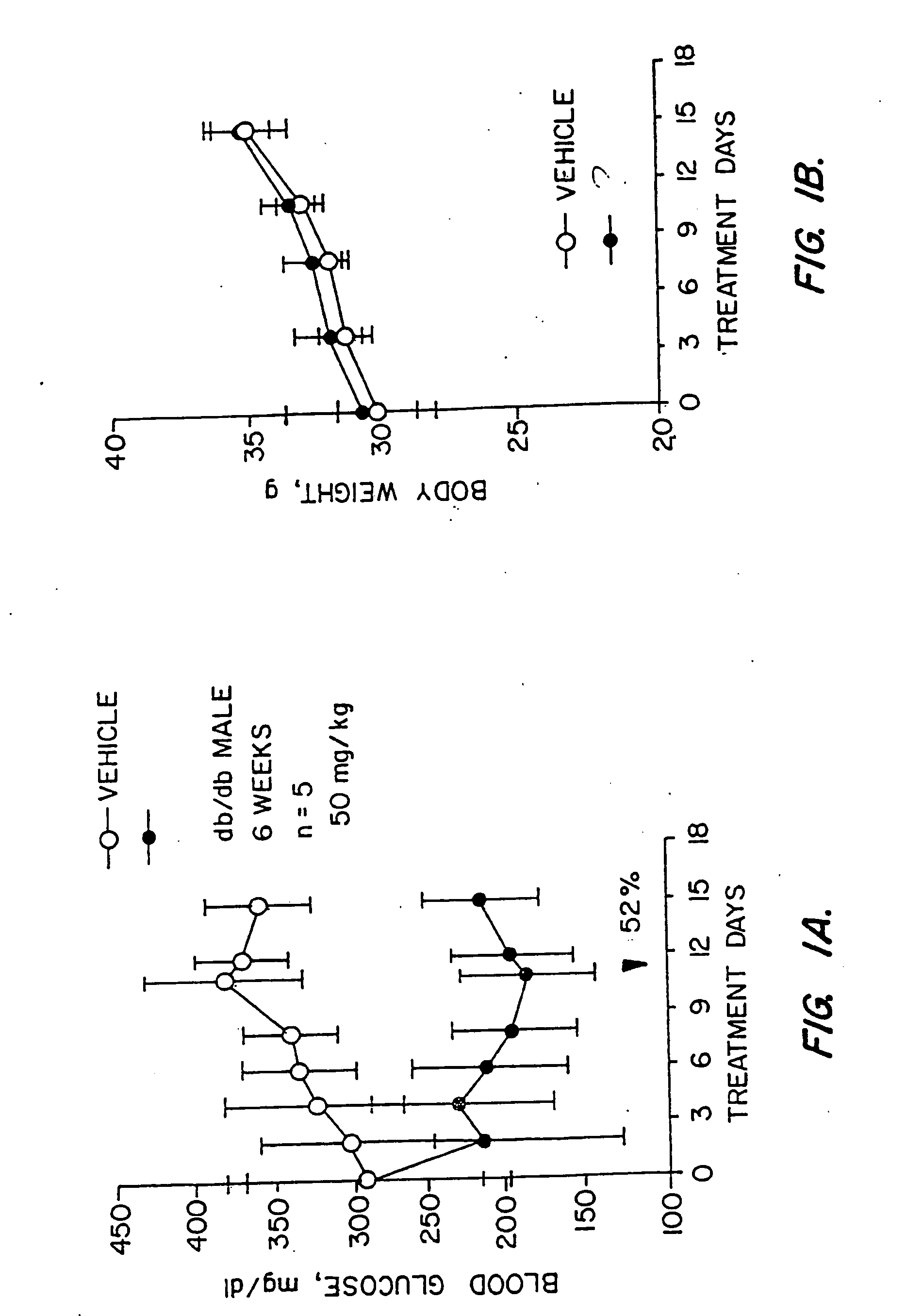
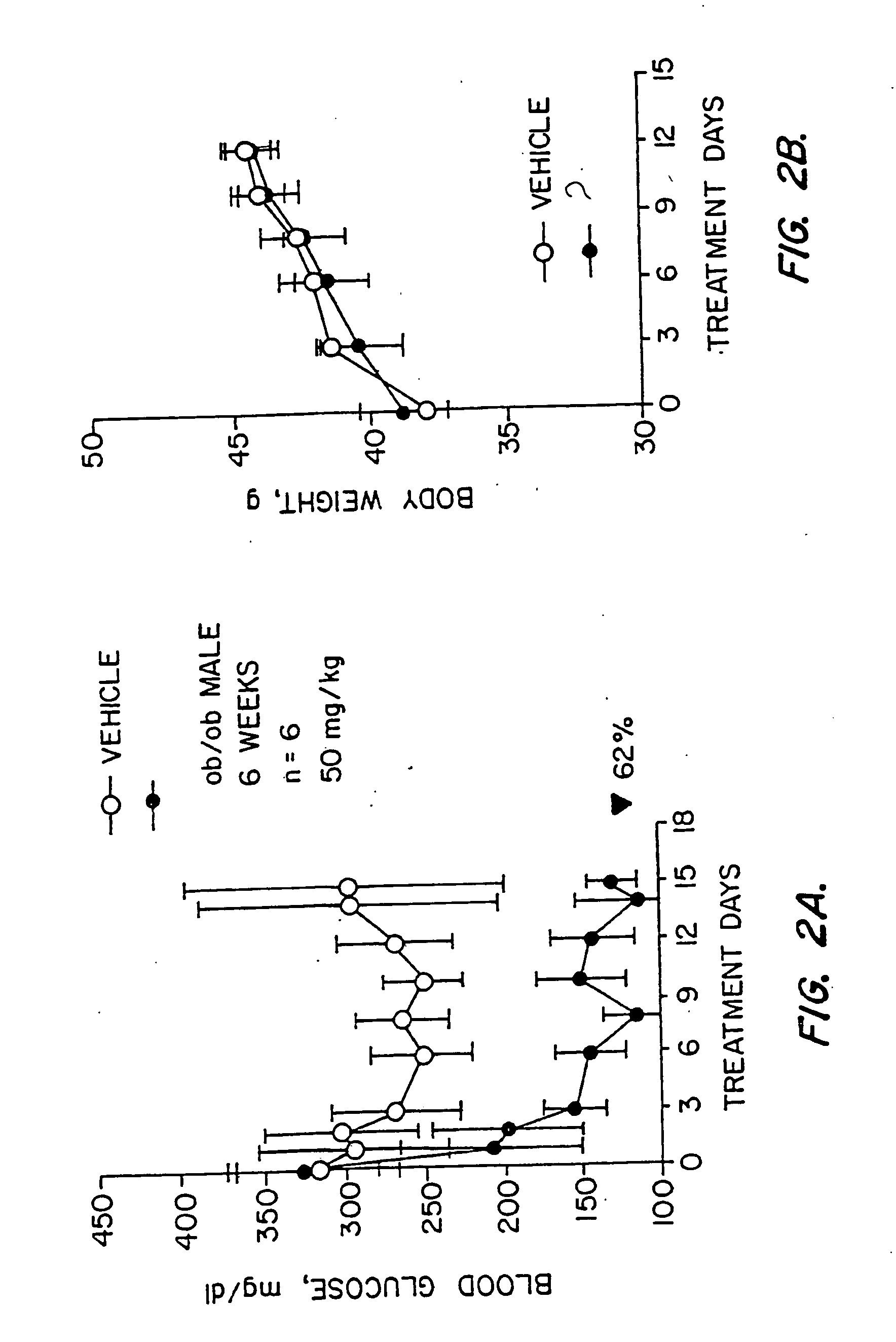
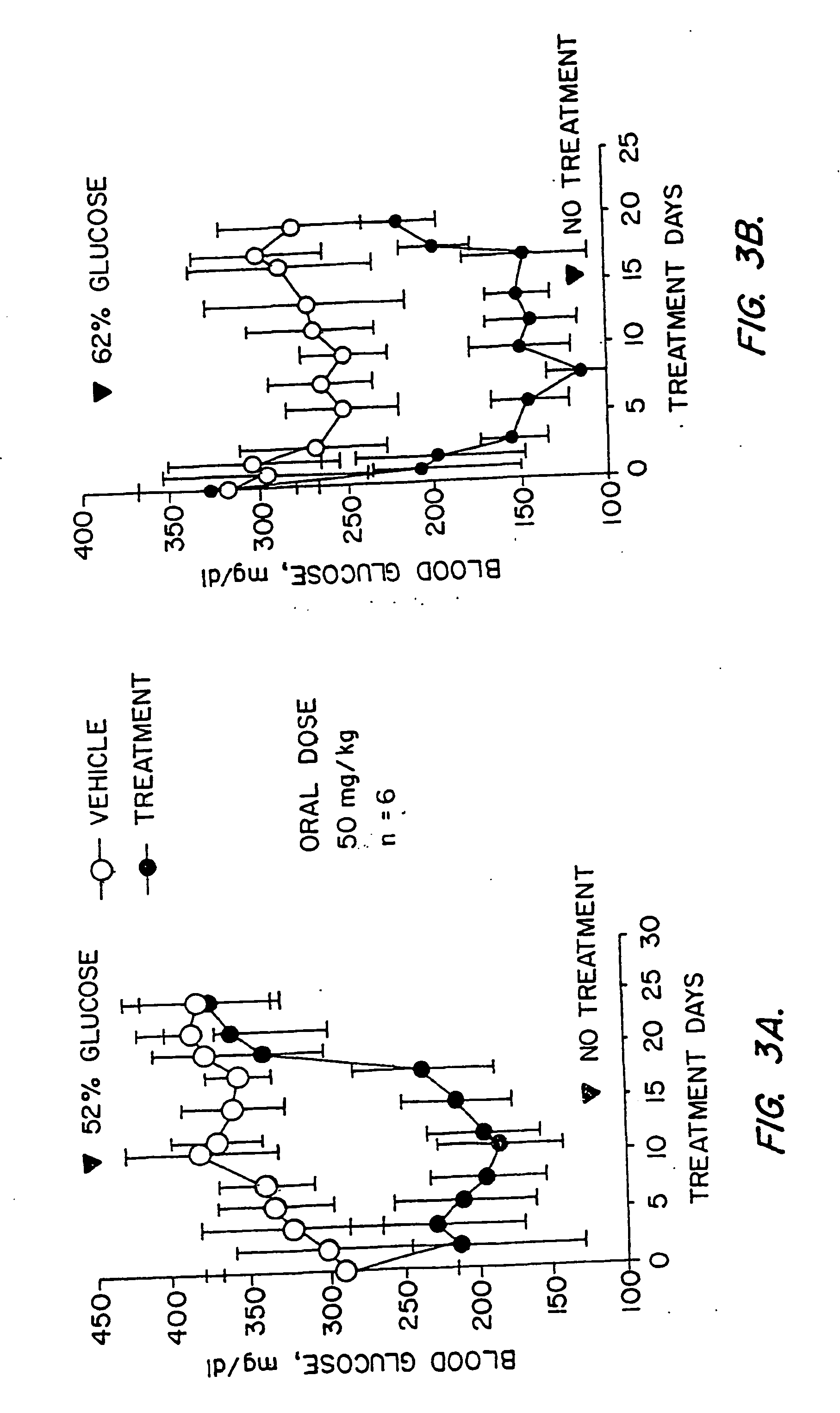
Novel heterocyclic analogs of diphenylethylene compounds
a diphenylethylene and heterocyclic technology, applied in the field of new heterocyclic analogs of diphenylethylene compounds, can solve the problems of morbidity and mortality, undesirable side effects of gastrointestinal pathology, and the ineffectiveness of known thiazolidinediones for a significant portion of the patient population
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0117] General Methods. Melting points were measured on a Mel-Temp melting point apparatus and are uncorrected. The 1H NMR and 13C NMR spectra were recorded on a JEOL Eclipse (400 MHz) or Nicolet NT 36 (360 MHz) spectrometer and are reported as parts per million (ppm) downfield from TMS. The infrared spectra were recorded on a Nicolet Impact 410 FT-IR spectrophotometer. The mass spectra were recorded on Fison VG Platform II of HP 1100 MDS 1964A mass spectrophotometer. UV spectra were recorded on a Beckman DU650 spectrophotometer. TLC was done on Merck silica gel F254 precoated plates. The silica gel used for column chromatography was ‘Baker’ silica gel (40 μm) for flash chromatography.
[0118] 3-(3,5-Dimethoxyphenyl)-2-(4-hydroxyphenyl)-acrylic acid (7). To a mixture of 3,5-dimethoxybenzaldehyde, 5, (500 g, 3.0 mol) and 4-hydroxyphenyl acetic acid, 6, (457 g, 3.0 mol) was added acetic anhydride (1.0 L, 10.60 mole) and triethylamine (420 mL, 3.0 mol). After stirring at 130-140° C. for...
example 2
3-(3,5-Dimethoxyphenyl)-2-{4-[4-(2,4-dioxothiazolidin-5-ylmethyl)-phenoxy]-phenyl}-acrylamide (24)
[0157] Compound 24, which may be represented by the formula:
was prepared as follows from compound 14. A clean dry flask with stirbar was charged with compound 14 (0.423 g, 0.837 mmol) and dry DMF (10 mL). Then with stirring carbonyldiimidazole (0.271 g, 1.67 mmol) was added and the reaction was heated to 60° C. for 1 h. while vented through an oil-bubbler. The reaction mixture was then cooled to 0° C. and 2M ammonia in methanol (2.1 mL, 4.2 mmol) was added. The reaction was worked up by partitioning the mixture with 10% citric acid (10 mL), ethyl acetate (50 mL), and water (40 mL). The organic phase was then rinsed sequentially with water (2×30 mL), brine (1×20 mL) and dried with anhydrous MgSO4. Concentration of the organics afforded crude product. The crude product was purified by silica gel chromatography using ethyl acetate-hexanes (1:1) containing 1% acetic acid to ethyl acetat...
example 3
3-(3,5-Dimethoxyphenyl)-2-{4-[4-(2,4-dioxothiazolidin-5ylmethyl)-phenoxy]-phenyl}-N,N-dimethylacrylamide (25)
[0158] Compound 25, represented by the formula:
was prepared as follows: A clean dry flask with stirbar was charged with compound 14 (0.422 g, 0.835 mmol) and dry DMF (1 mL). Then with stirring carbonyldiimidazole (0.271 g, 1.67 mmol) was added and the reaction was heated to 60° C. for 1 h. while vented through an oil-bubbler. The reaction mixture was then cooled to 0° C. and a 2M dimethylamine in THF (2.1 mL, 4.2 mmol) solution was added. The reaction was worked up by partitioning the mixture with 10% citric acid (10 mL), ethyl acetate (50 mL), water (40 mL). The organic phase was then rinsed sequentially with water (2×30 mL), brine (1×20 mL) and dried with anhydrous MgSO4. Concentration of the organics afforded crude product. The crude product was purified by silica gel chromatography using ethyl acetate-hexanes (3:2) containing 1% acetic acid elution. Concentration of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com