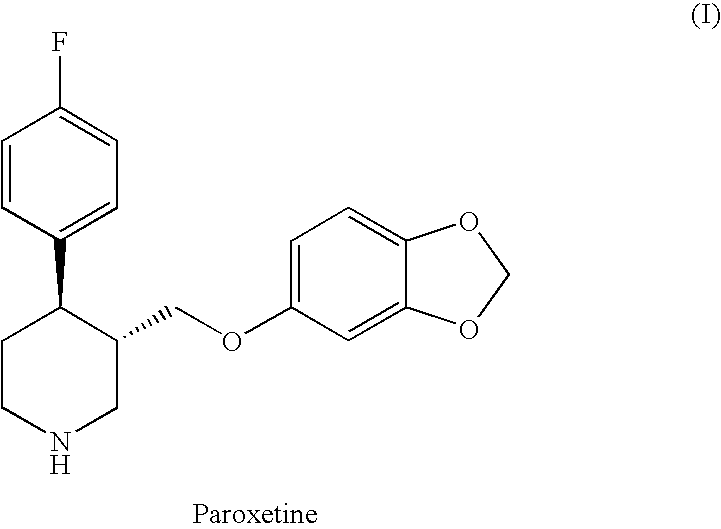
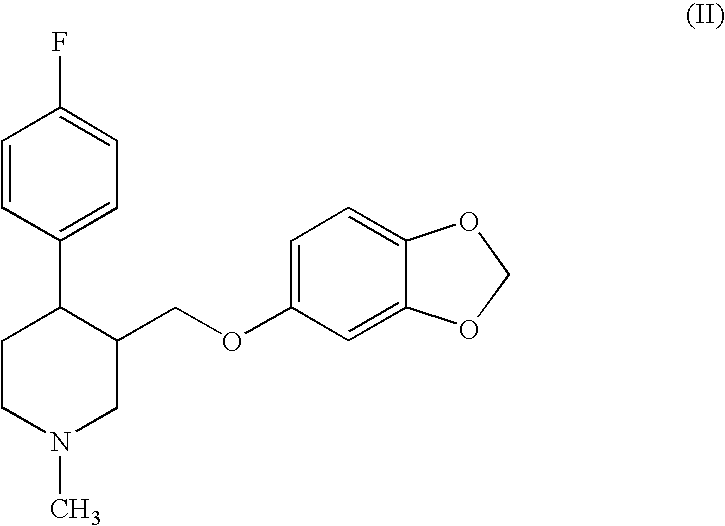
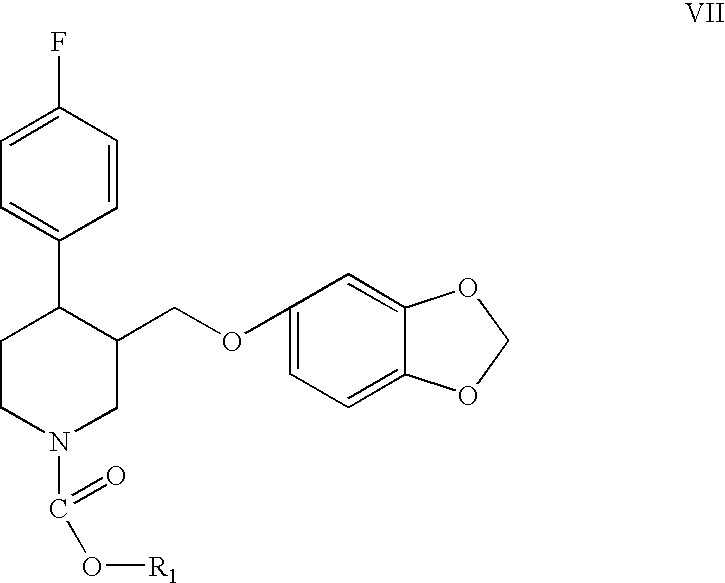
Preparation of paroxetine involving novel intermediates
a technology of intermediates and paroxetine, which is applied in the field of intermediate synthesis processes, can solve the problems of large quantities of phenol as undesirable by, low yield of paroxetine, and long time required for the conversion of me-prx to paroxetin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Reaction with 2-Cl-ethylchloroformate (CECF) in dry conditions
[0050] Me-PRX (3 g) and extra dry toluene (40 ml, less than 0.001% water) are charged into dried equipment under a dry N2 stream. The reaction mixture is cooled to 4° C. with an ice bath. CECF (2.7 mL, 3 eq., purchased from SNPE) is added dropwise for several minutes. The mixture is heated to reflux for 7 hours providing the substantially complete conversion of Me-PRX (HPLC) to the carbamate. Water (50 mL) is added to cool the reaction mixture to room temperature. The organic layer is separated, washed with water, dried with Na2SO4 and evaporated to dryness to give 1-(2-chloroethoxycarbonyl)-4-fluorophenyl-3-[5-(1,3-dioxaindanyl)oxymethyl]piperidine, i.e., the 2-chloroethyl carbamate of paroxetine (CECB).
example 2
Reaction with CECF in the presence of Bu3N
[0051] The same procedure as described in Example I is repeated, except that the equipment is not previously dried, technical grade toluene (less than 0.10% water) is used instead of extra dry toluene, and the reactants are not charged under a dry N2 stream. The reaction mixture, before addition of CECF, also contains 2.05 g (1.2 eq.) of Bu3N. After 1.5 hours of reflux, substantially complete conversion of Me-PRX to the corresponding carbamate takes place. The carbamate, i.e., the 2-chloroethyl carbamate of paroxetine (CECB) is separated from the reaction mixture using the same procedures as described in Example 1.
example 3
Reaction with CECF in the presence of Et3N
[0052] The same procedure as described in Example 2 is repeated, but with 1.1 g Et3N (1.2 eq.) used in place of Bu3N. After 4 hours of reflux, conversion of the Me-PRX to the corresponding carbamate is 74% (reaction stopped).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com