Vectors and methods for gene transfer
a technology of vectors and gene transfer, applied in the field of vectors and methods for gene transfer, can solve the problems of preventing effective re-administration of viral vectors, affecting the efficiency of adenovirus-mediated gene delivery, and affecting the efficiency of gene entry, so as to achieve the effect of increasing the efficiency of entry and low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
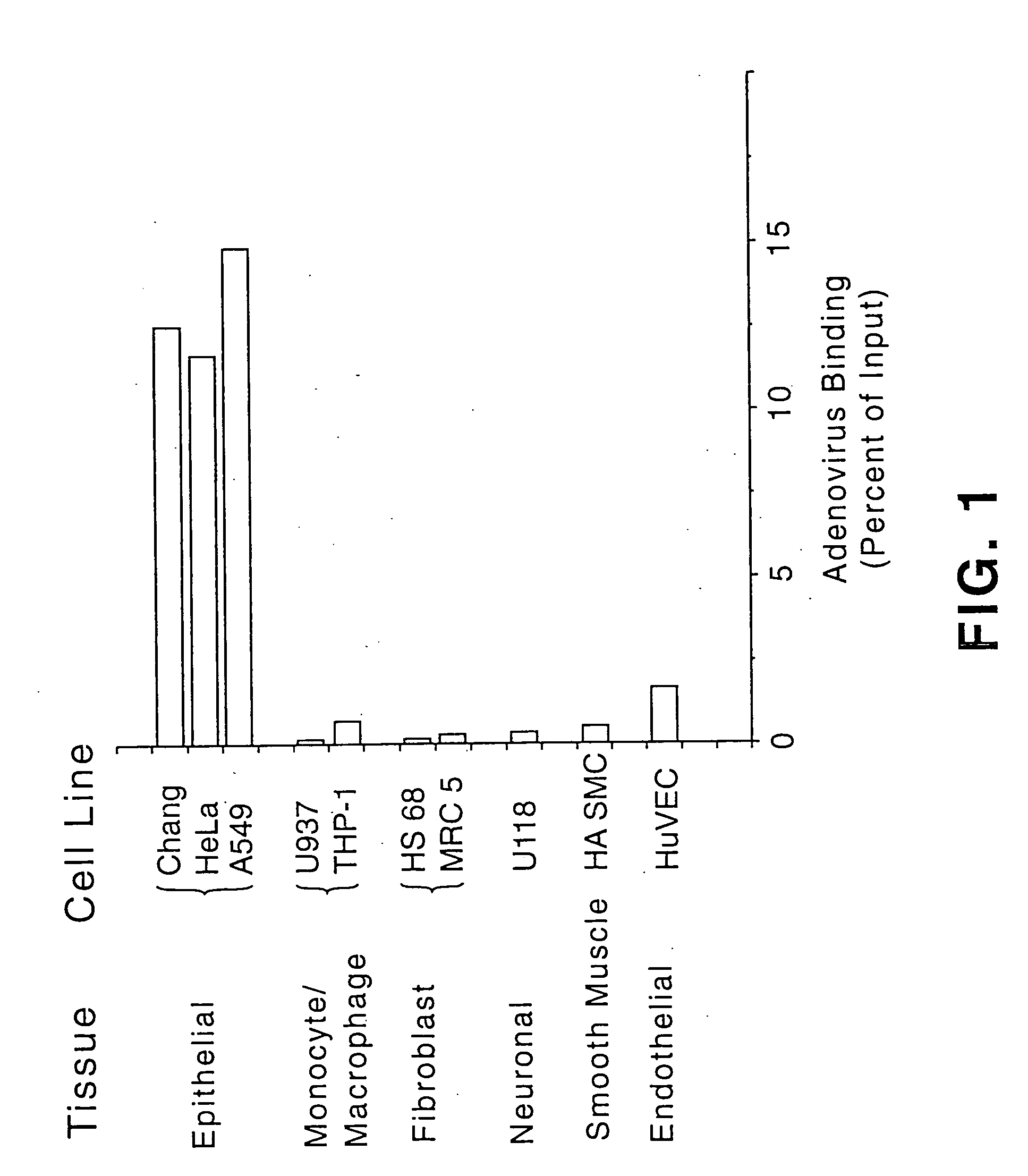
[0118] This example describes an investigation of the levels of adenovirus receptor in different cells, as determined by the ability of wild-type adenovirus to bind to the cells.
[0119] For these experiments, the ability of adenovirus comprising wild-type fiber to bind to cells derived from various tissues was assessed. Adenovirus particles of an Ad5 strain were labeled with [3H]-thymidine as previously described (see, e.g., Wickham et al., Cell, 73, 309-319 (1993)). Subsaturating levels of thymidine-labeled adenovirus were added to 200 μl of 106 cells preincubated about 30 to 60 minutes with or without 20 μg / ml of soluble fiber protein. The cells were incubated with the virus for 1 hour at 4° C. and then washed 3 times with cold phosphate buffered saline (PBS). The remaining cell-associated counts were measured in a scintillation counter. Specific binding was measured by subtracting the cell-associated counts (i.e., counts per minute (cpm)) in the presence of fiber from the cell-as...
example 2
[0122] This example describes the construction of an adenoviral vector comprising a chimeric coat protein, particularly a chimeric adenoviral fiber protein.
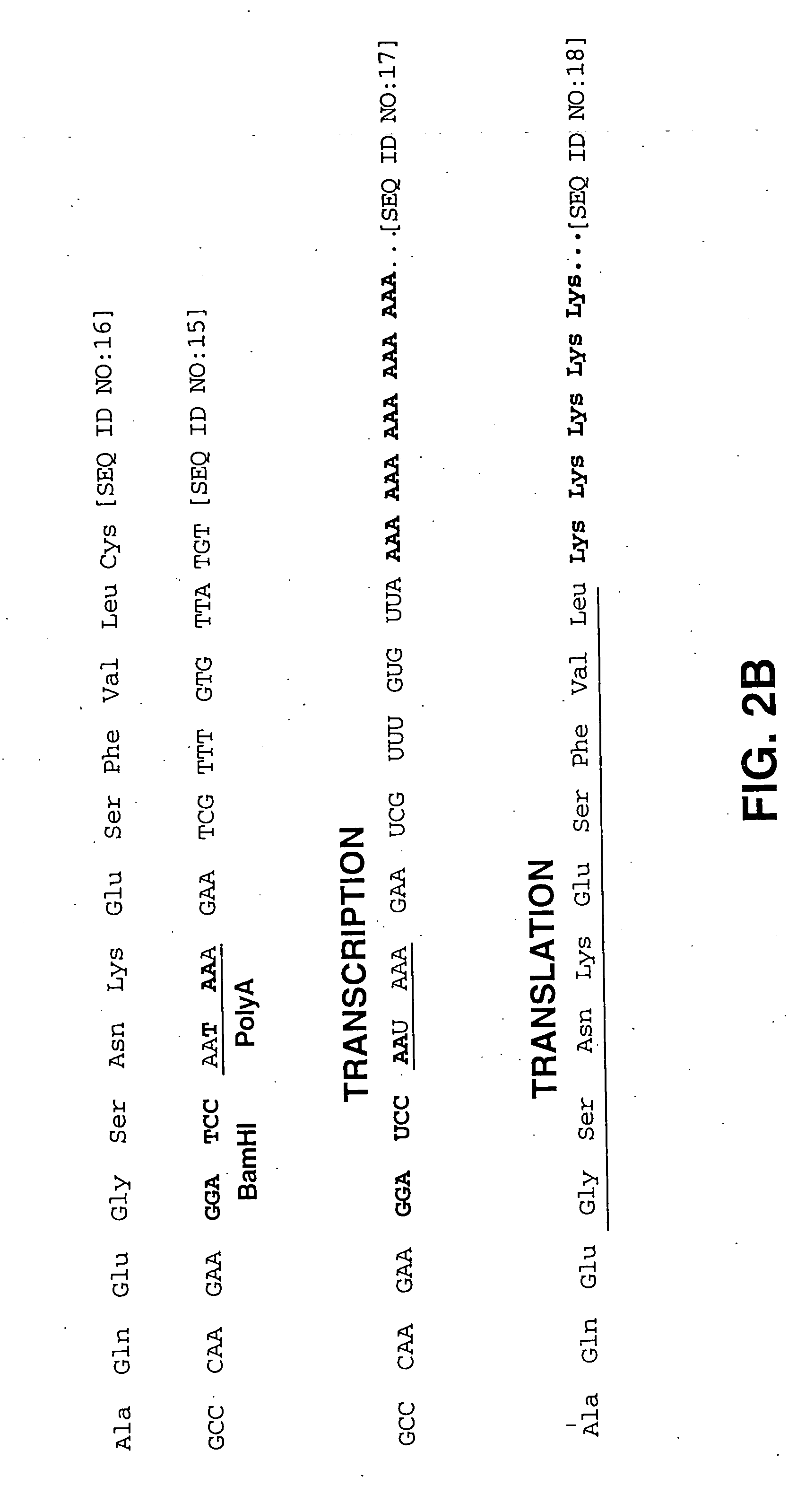
[0123] To overcome the transduction limitation imposed by the presence of only a limited number of fiber receptors on clinically relevant tissues such as non-epithelial tissue, a modified adenovirus vector was constructed as depicted in FIGS. 2A and 2B to derive a vector that is referred to herein as a “universal transfer vector”, or UTV. In particular, a frameshift mutation was created in a gene encoding an adenoviral coat protein, in this case, in the fiber gene. In wild-type adenovirus, the unmodified fiber gene contains a nested translational stop signal (TAA) and transcriptional polyadenylation signal (AATAAA). The polyadenylation signal directs the addition of a polyA tail onto the 3′ end of the transcript. The polyA tail typically comprises anywhere from about 20 to about 200 nucleotides. Following transcription and exit ...
example 3
[0136] This example describes the binding to cells of an adenoviral vector comprising a chimeric coat protein such as a chimeric fiber protein as compared with a wild-type adenoviral vector, either in the presence or absence of added soluble wild-type fiber protein
[0137] For these experiments, the cells identified in Example 1 to which adenovirus binds with either high efficiency (i.e. receptor-plus cells) or low efficiency (i.e., receptor-minus cells) were employed. The epithelial cell line A549 was used as representative of receptor-plus cells, and the fibroblast cell line HS 68 was used as representative of receptor-minus cells. Confluent monolayers of either A549 or HS 68 cells were preincubated at 4° C. with concentrations of soluble fiber protein ranging from 0 to about 10 μg / ml. The GV10 UTV vector comprising chimeric fiber protein (UTV) or GV10 vector comprising wild-type fiber protein (WT) were labeled with tritiated thymidine as described in Example 1. About 20,000 cpm of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com