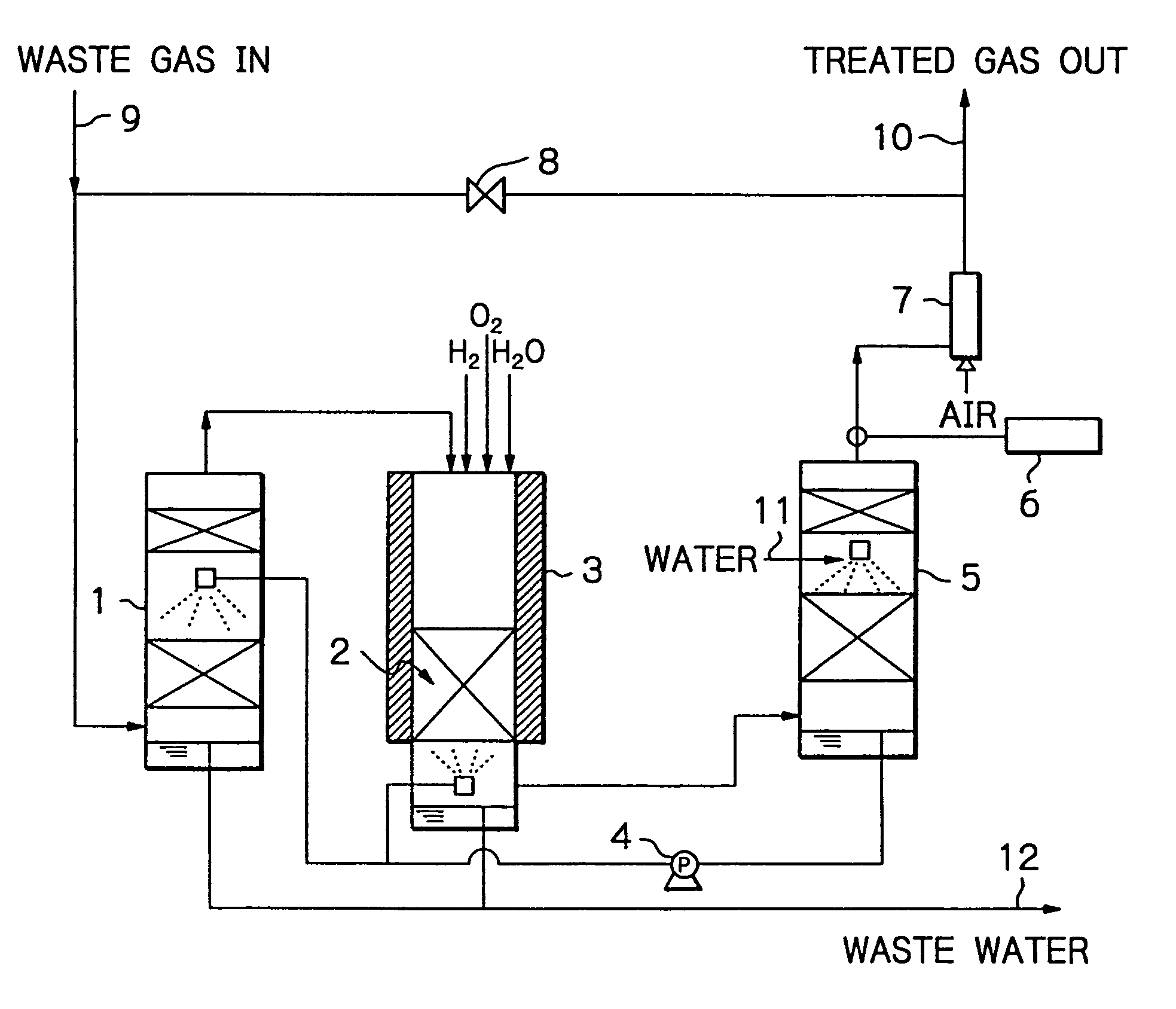
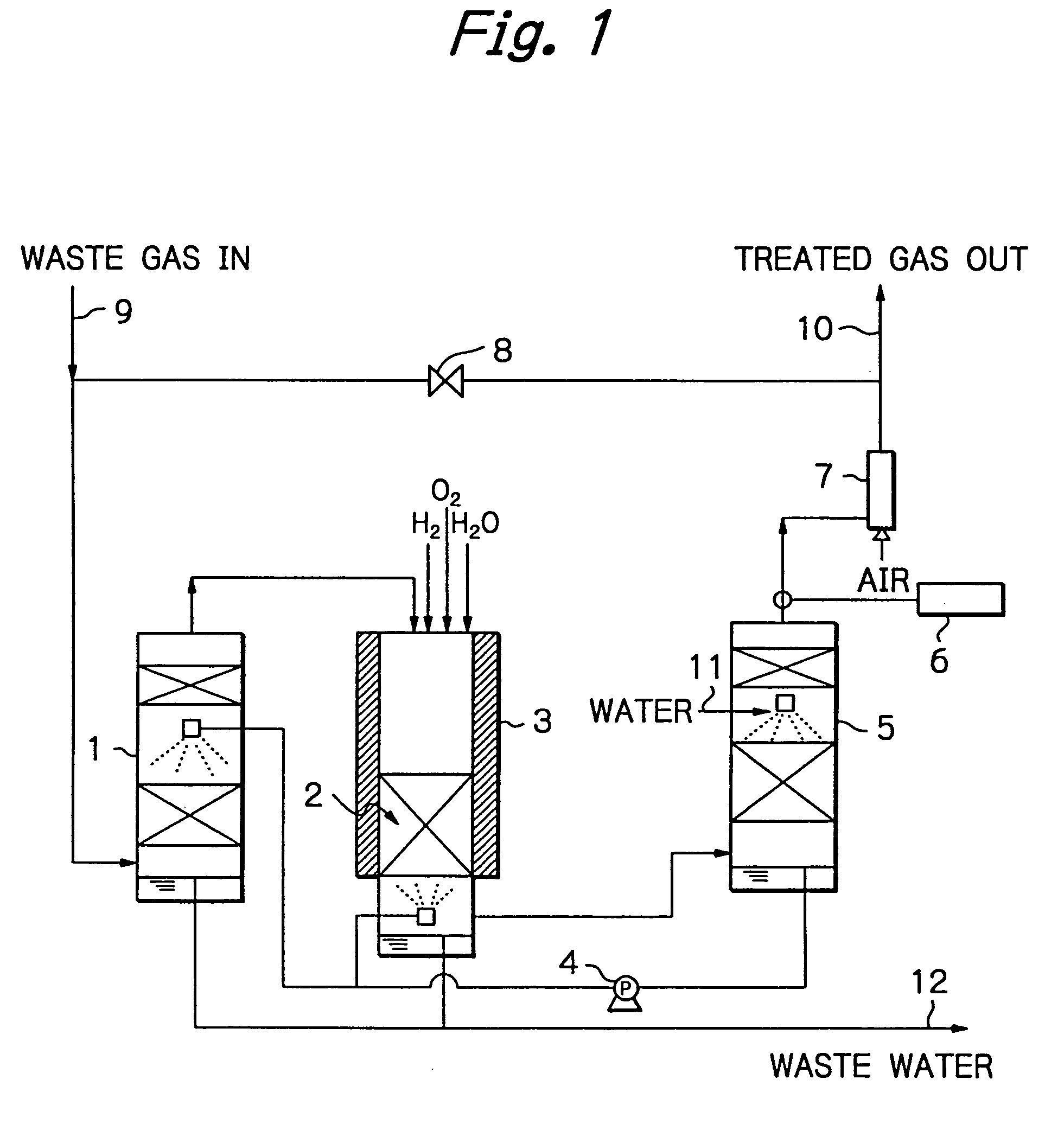
Method and apparatus for treating a waste gas containing fluorine-containing compounds
a technology of fluorine-containing compounds and waste gas, which is applied in the direction of machine/engine, physical/chemical process catalysts, separation processes, etc., can solve the problems of unsatisfactory catalytic compounds, no method has been established to realize a thorough and effective treatment of these harmful gases, and the effect of high decomposition of pfcs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0028] An experiment was conducted using a quartz column of 25 mmφ, which was packed with γ-alumina to a height of 100 mm. The γ-alumina was a commercial product of Mizusawa Kagaku K.K. (NEOBEAD GB-08) having a particle size of 0.8 mm. The quartz column was installed in a ceramic electric furnace and the γ-alumina was heated at 800° C.
[0029] In addition to CF4 diluted with N2 gas, H2 and O2 were supplied as decomposition assist gases, with the amount of H2 being such that the number of H atoms was at least equal to the number of F atoms in CF4, and the amount of O2 being at least equimolar to the amount of H2 supplied. These gases were flowed into the column at a total rate of 408 sccm and their entrance concentrations were 1.0% (CF4), 3.0% (H2) and 5.7% (O2).
[0030] In order to evaluate performance of the treatment system, exit gas was analyzed periodically and passage of the CF4, gas was stopped when removal of CF4 dropped below 98%. Throughput was determined from the amount of C...
example 2
[0034] An experiment was conducted using the same equipment as in Example 1, which was packed with the same γ-alumina in the same amount and heated to the same temperature as that of Example 1. Total gas flow rate was 408 sccm; feed gas was a mixture of N2-diluted CF4 and F2; in addition, H2 and O2 were supplied as decomposition assist gases, with the amount of H2 being such that the number of H atoms was at least equal to the total number of F atoms in CF4 and F2, and the amount of O2 being at least equimolar to the amount of H2 supplied. These gases were flowed into the column at respective concentrations of 0.92% (CF4), 1.1% (F2), 5.0% (H2) and 6.0% (O2).
[0035] As it turned out, the removal of CF4 dropped below 98% when passage of the CF4 / F2 gas continued for 25 hours. At this point in time, throughput was 115 L / L, which was 1.51 times higher than the throughput for the case where only CF4 gas was supplied. Throughout the experiment, concentrations of CO and F2 were below tolera...
reference example 1
[0036] An experiment was conducted using the same equipment as in Example 1, which was packed with the same γ-alumina in the same amount and heated to the same temperature as that of Example 1. The total gas flow rate was 408 sccm; in addition to N2-diluted CO, O2 was supplied in moles at least equal to the moles necessary for CO to be converted into CO2, and their respective entrance concentrations were 1.4% (CO) and 5.7% (O2). Throughout passage of a feed gas for 30 minutes, concentration of CO was below the detection limit (2 ppm), and all of CO had been oxidized into CO2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com