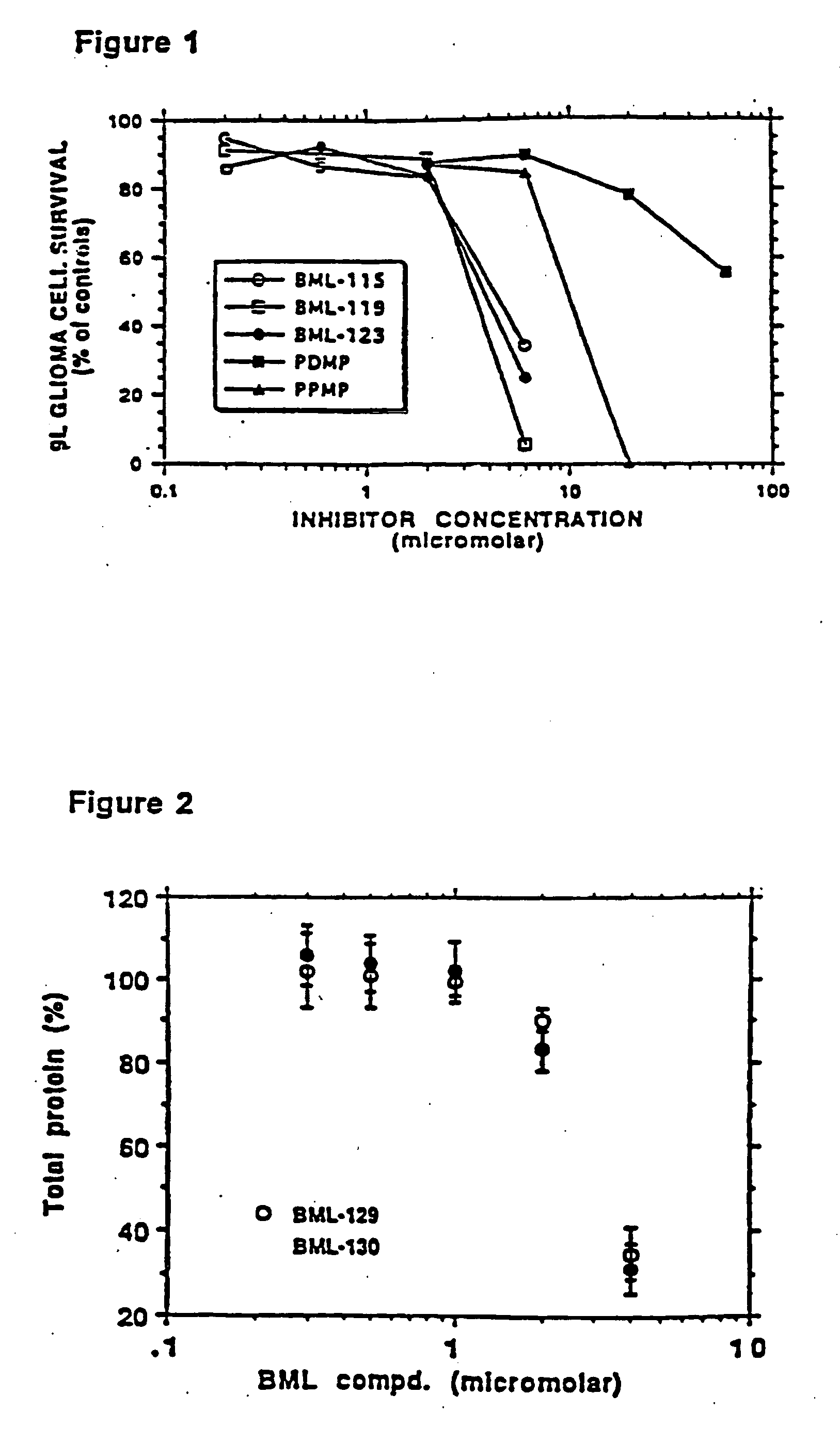
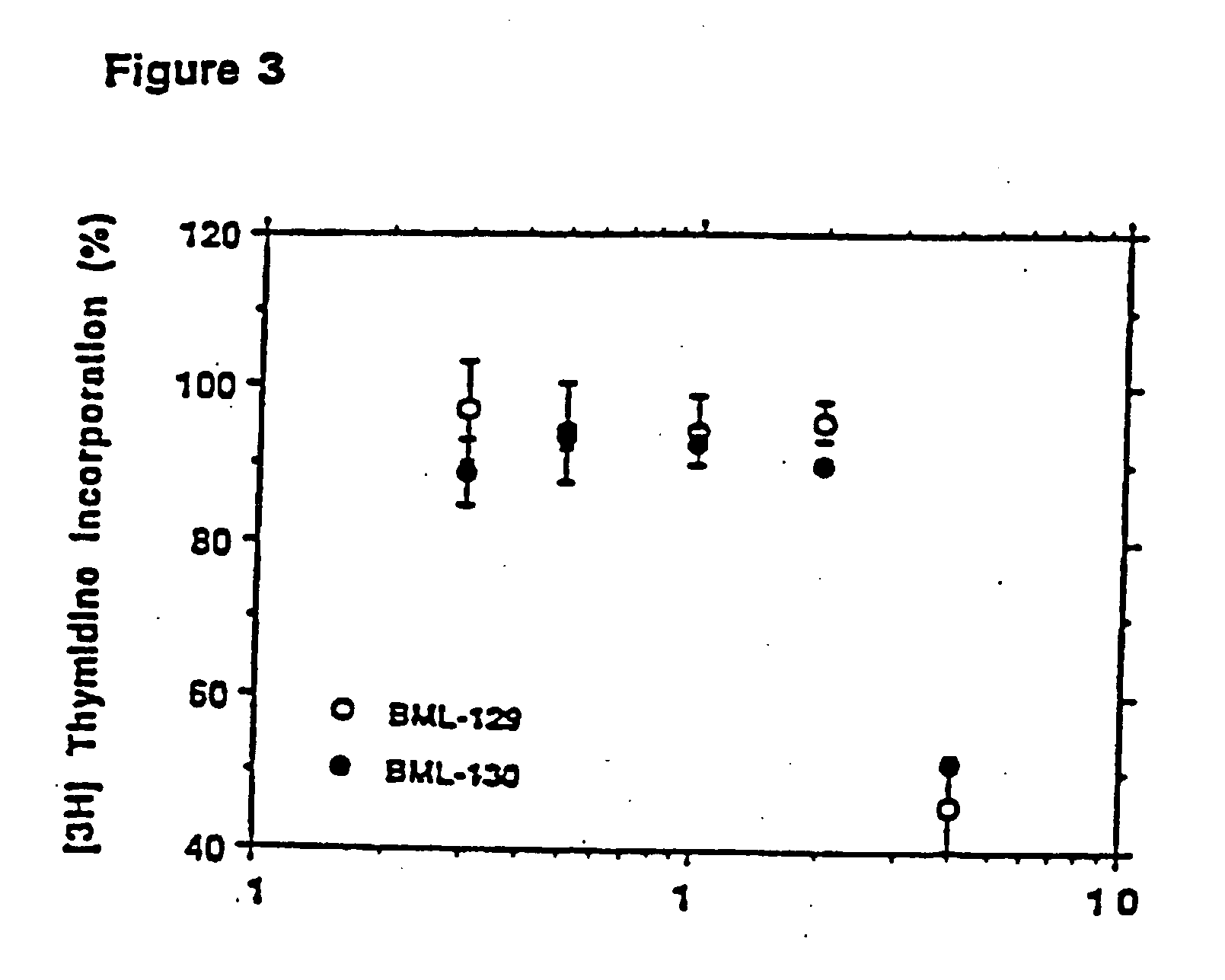
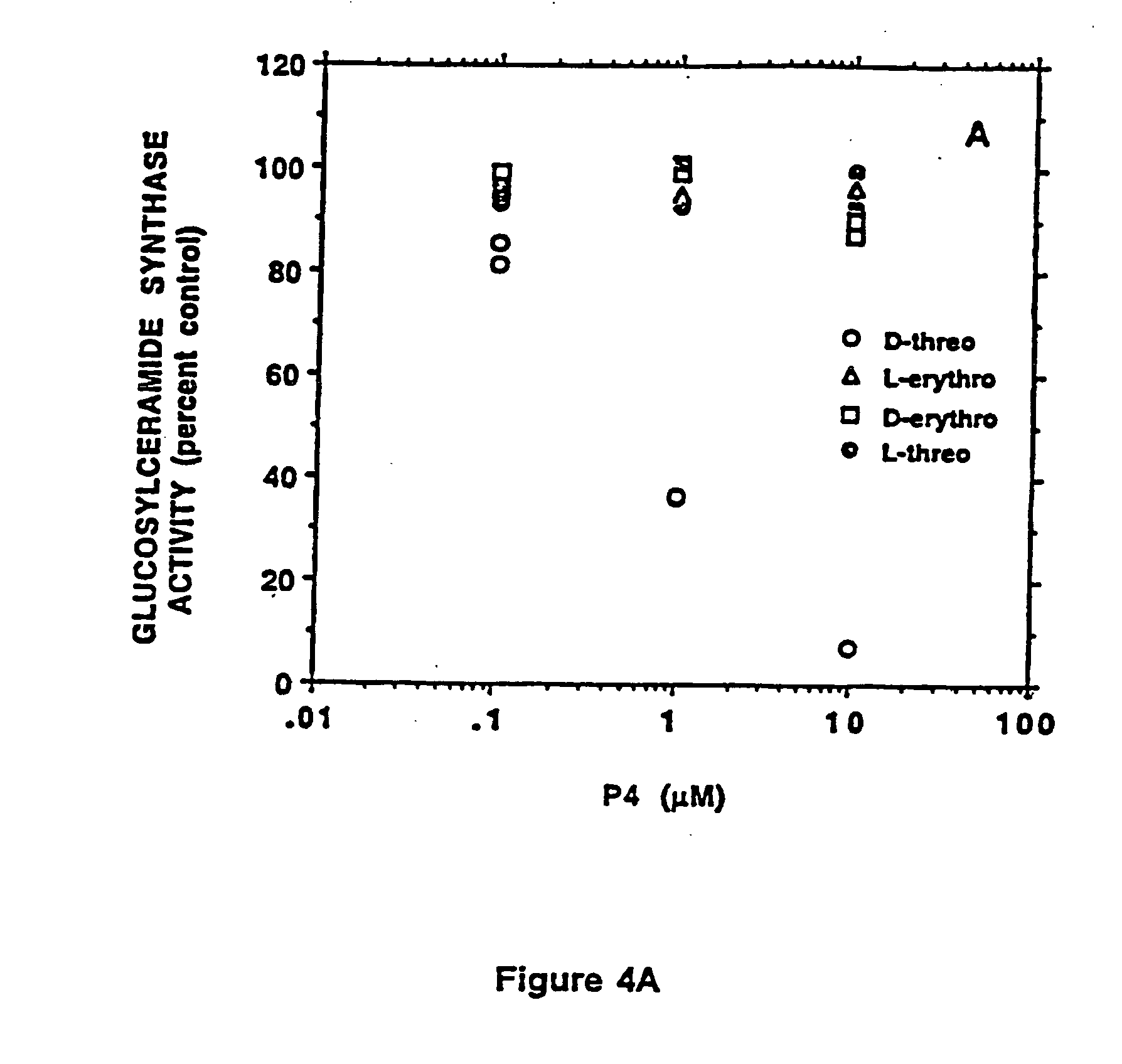
Amino ceramide-like compounds and therapeutic methods of use
a technology of ceramide and ceramide, which is applied in the field of ceramidelike compounds, can solve the problems of high treatment cost, hundreds of thousands, or even millions of dollars, over a patient's lifetime, and achieve the effects of reducing the level of gsls, reducing the risk of glccer formation, and improving the activity of glccer synthase inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific example 1
[0053] The following formulas set forth preferred aromatic and aliphatic compounds:
identified as (1R,2R)-1-phenyl-2-acylamino-3cylic amino-1-propanol, and referred to herein as the “aromatic inhibitors,” wherein
[0054] The phenyl group can be a substituted phenyl group (such as p-methoxyphenyl). [0055] R′ is an alkyl residue of a fatty acid, 10 to 18 carbons long. The fatty acid can be saturated or unsaturated, or possess a small substitution at the C-2 position (e.g. a hydroxyl group). [0056] R is morpholino, pyrrolidino, piperidino, azetidino (trimethyleneimino), N-methylethanolamino, diethylamino or N-phenylpiperazino. A small substituent, such as a hydroxyl group, is preferably included on the cyclic amine moiety.
identified as (2R,3R)-2-palmitoyl-sphingosyl amine or 1-cylic amino-1-deoxyceramide or 1-cyclic amino-2-hexadecanoylamino-3-hydroxy-octadec4,5-ene, and referred to herein as the “aliphatic inhibitors,” wherein [0057] R′ is an alkyl residue of a fatty acid, 10 to 1...
specific example 2
[0105] A series of inhibitors based on substitutions in the phenyl ring of P4 were synthesized and studied. It was found that the potency of the inhibitors in blocking GlcCer synthase was mainly dependent upon hydrophobic and electronic properties of the substituent. Surprisingly, a linear relationship was found between log [IC50] and hydrophobic parameter (π)+electronic parameter (δ). This correlation suggested that electron donating and hydrophilic characters of the substituent enhance the potency as an inhibitor. This observation resulted in the synthesis of novel compounds that are more active in blocking glucosylceramide formation. Two compounds, dioxy D-t-P4 compounds, D-t-3′,4′-ethylenedioxy-P4 and D-t-4′-hydroxy-P4, were observed to be significantly more potent than other tested inhibitors. In particular, at 11.3 nM D-t-3′,4′-ethylenedioxy-P4, 80% of glucosylceramide in MDCK cell was depleted without any ceramide accumulation and cell growth inhibition. The potency of D-t-3′...
specific example 3
[0139] Compositions within the scope of invention include those comprising a compound of the present invention in an effective amount to achieve an intended purpose. Determination of an effective amount and intended purpose is within the skill of the art. Preferred dosages are dependent for example, on the severity of the disease and the individual patient's response to the treatment
[0140] As used herein, the term “pharmaceutically acceptable salts” is intended to mean salts of the compounds of the present invention with pharmaceutically acceptable acids, e.g., inorganic acids such as sulfuric, hydrochloric, phosphoric, etc. or organic acids such as acetic.
[0141] Pharmaceutically acceptable compositions of the present invention may also include suitable carriers comprising excipients and auxiliaries which facilitate processing of the active compounds into preparations which may be used pharmaceutically. Such preparations can be administered orally (e.g., tablets, dragees and capsu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com