Delivery and recovery system for embolic protection system
a technology of embolism protection and delivery system, which is applied in the field of improving the efficiency of the delivery and recovery of emboli, can solve the problems of affecting the patient's health, affecting the patient's recovery, so as to achieve the effect of a higher degree of confidence in the efficient delivery and recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
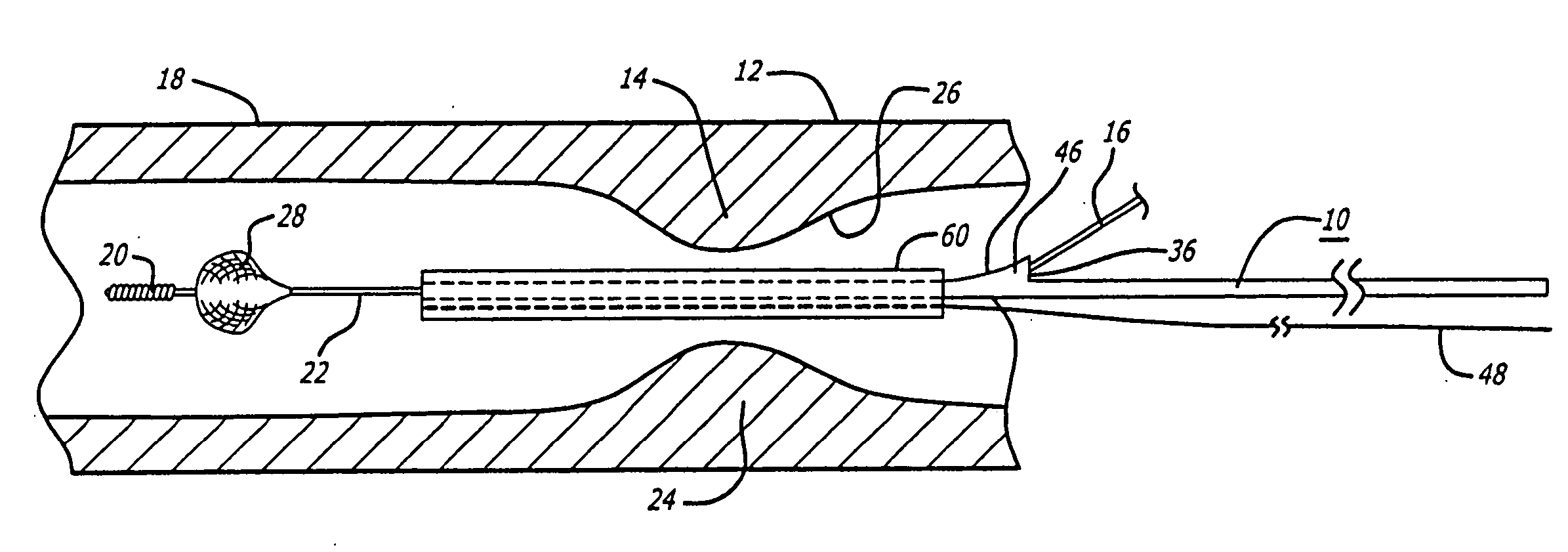
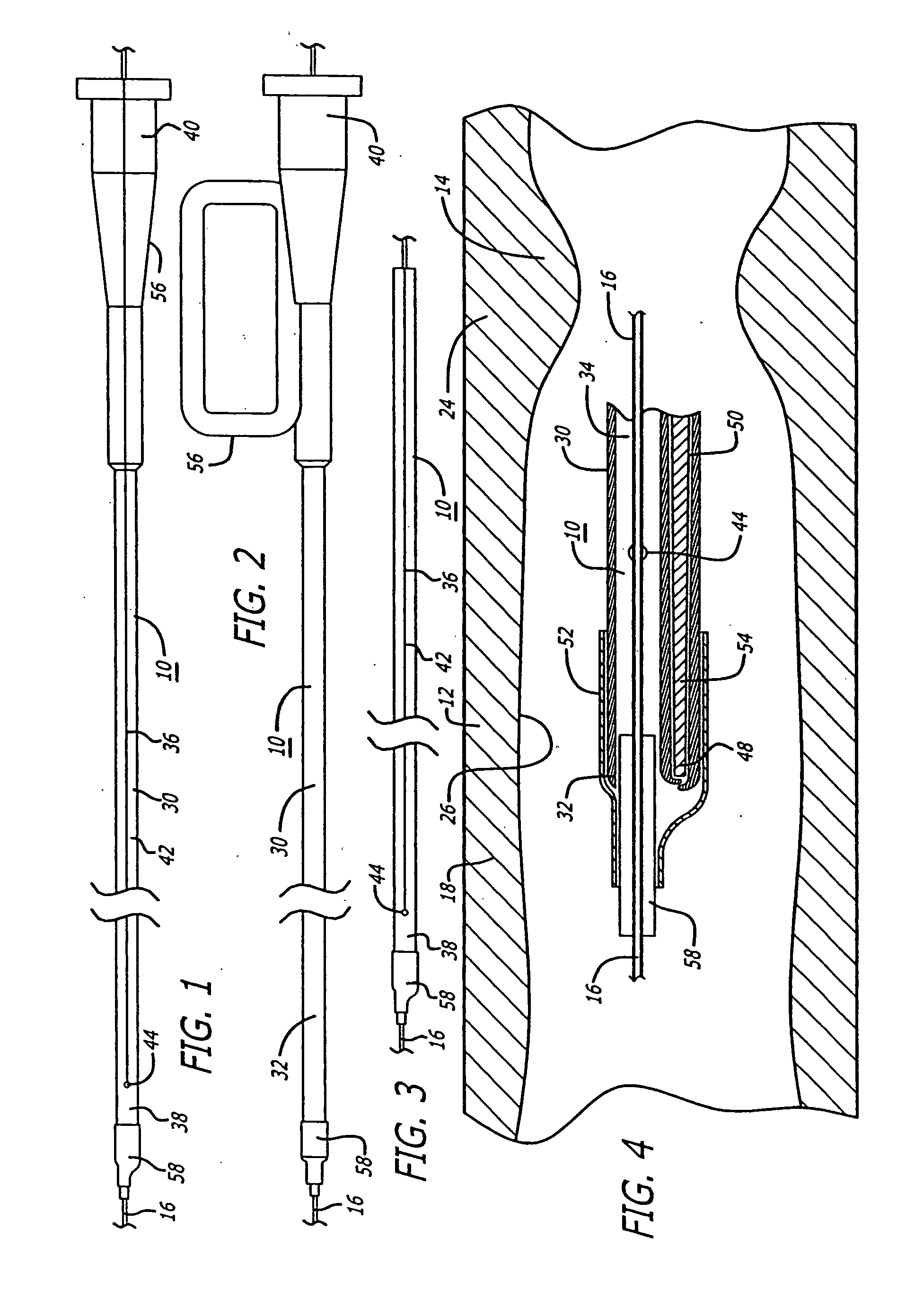
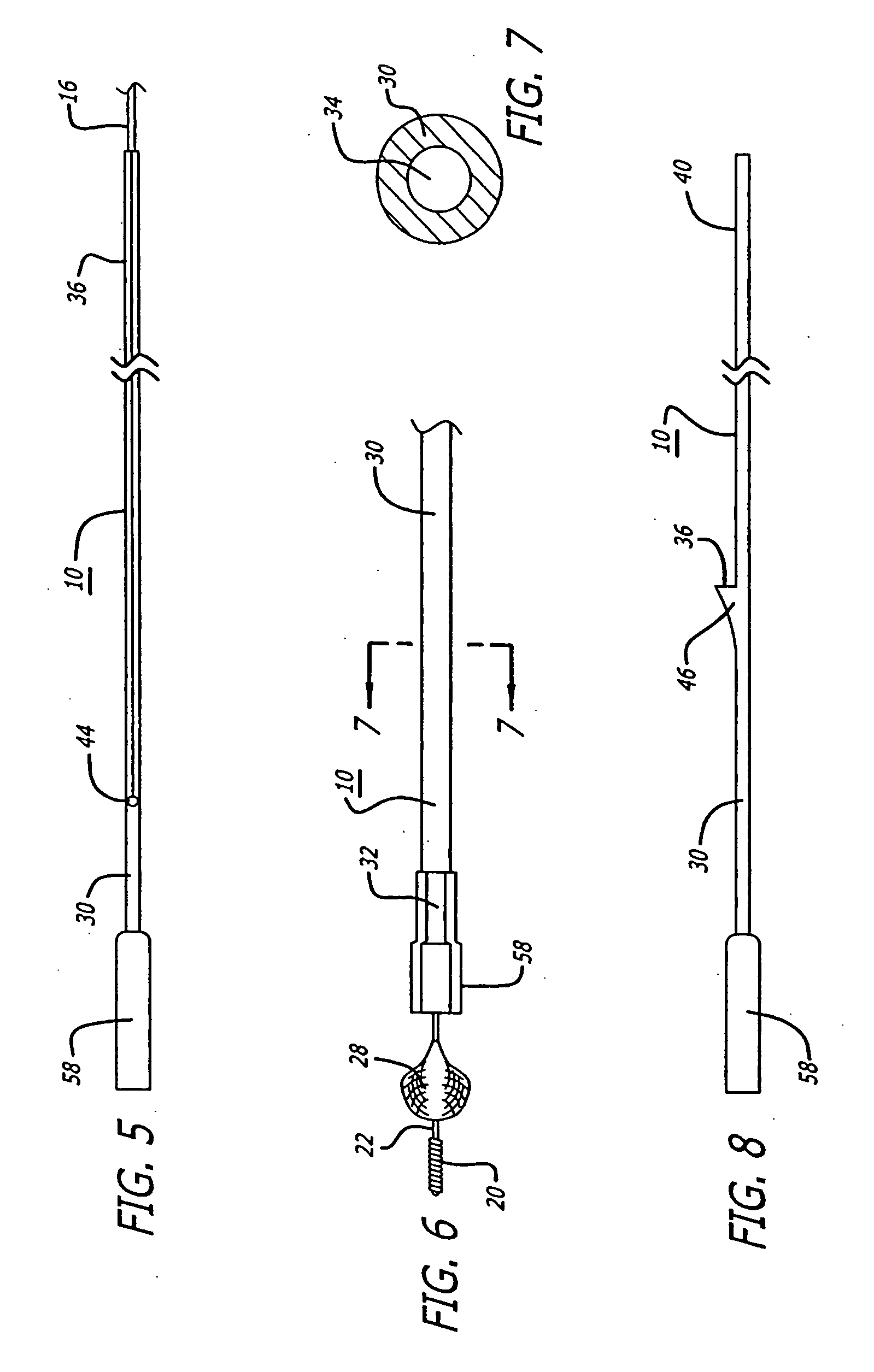
[0058] Referring to FIGS. 1-18, in a system pursuant to the present invention, for example, the system 10 is adapted to enable at least one operator to control of the delivery of the embolic protection device 28 to the position in a patient's blood vessel 12 distal to the area of treatment 14, for deployment of the embolic protection device 28. The system 10 is further adapted to enable the at least one operator to control the removal of the delivery system 10, to enable the exchange of the delivery system, and to enable the control of the position of a deployed embolic protection device 28 within the patient's vasculature 12 during an exchange of interventional devices. The system 10 is further adapted to enable the at least one operator to control the removal of the embolic protection device 28 through the patient's vasculature 12, from the delivered and deployed position thereof, for the exchange of the recovery system.
[0059] As illustrated in FIGS. 1-9, in a delivery version of ...
second embodiment
[0068] Referring to FIGS. 19-26, in a system pursuant to the invention, for example, a system 64 is provided for enabling the delivery and recovery of an embolic protection device 28 relative to a position in the patient's vasculature 12 distal to an interventional procedure site 14, through the patient's vasculature 12, for deployment of the embolic protection device 28. The system 64 is adapted to maintain a clinically acceptable profile and flexibility during the delivery and removal thereof through the patient's vasculature 12. Elements of the system 64 are comprised of polymer materials such as for example PeBax which is comprised of a thermoplastic polyimide. Also, for the delivery of the embolic protection device 28, the materials are such as to provide substantial flexibility for enabling delivery thereof through the patient's anatomy and for preventing the guide wire 16 from kinking, while providing sufficient rigidity for enabling substantial pushing force to be exerted fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com