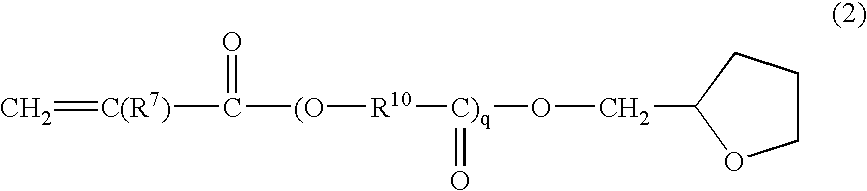
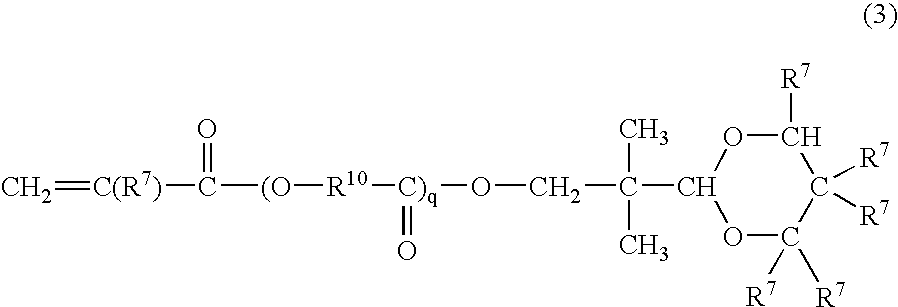
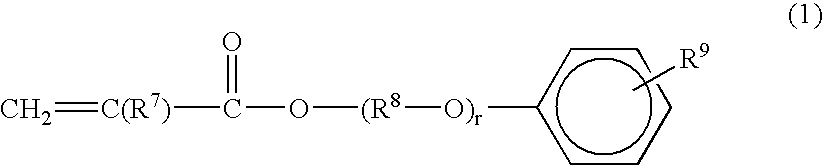Radiation curable resin composition
a technology of resin composition and cure speed, applied in the field of cureable composition, can solve the problems of impairing the handling of resin composition, the cure speed of coatings and/or binders, and the limit on the speed of production line operation, so as to improve the cure speed, improve the mechanical characteristics, and improve the effect of liquid stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 2 and 3
[0079] Components shown in Table 2 were mixed in the amounts noted (wt %).
[0080] Oligomer I is the reaction product of 5.87 wt % TDI, 2.6 wt % of 2-hydroxyethylacrylate (HEA) and 91.39 wt %. Acclaim 4200 (Mw: 4000; unsaturation of 0.003 meq / g), catalyst and stabilizer.
[0081] Oligomer II is the reaction product of 9.7 wt % IPDI, 3.37 wt % HEA, 57.15 wt % Acclaim 4200N and 29.62 wt % Priplast 3190. Priplast 3190 is a polyester polyol with dimer acid from Unichema.
[0082] Results are given in Table 3.
TABLE 2Example 2Example 3Oligomer I68.59—Oligomer II—77.10ENPA7.00—IDA— 8.50TriDa7.00—Ebecryl III5.00—VC4.00 5.00SR90034.00 5.00Lucerine TPO1.31.3Irgacure 1841.81.8Irganox 10350.30.3Silane1.01.0
ENPA: ethoxylated nonylphenol acrylate
IDA: isodecyl acrylate
TriDa: tridecyl acrylate
Ebecryl III: ethoxylated aliphatic acrylate from UCB
VC: N-vinylcaprolactam
SR9003: propoxylated neopentyl glycol diacrylate
Silane: γ-mercaptopropyl trimethoxysilane
[0083]
TABLE 3Example 2Example 3viscosity (...
examples 4 and 5
[0084] A reaction vessel equipped with a stirrer was charged with 53.34 g of 2,4-tolylene diisocyanate, 150 g of nonylphenol EO-modified (4 mols) acrylate (monofunctional acrylate “Aronix M-113” manufactured by Toagosei Co., Ltd.), 0.1 g of 2,6-di-t-butyl-p-cresol, and 0.4 g of dibutyltin dilaurate. The mixture was cooled with ice to a temperature of 10° C. or less while stirring. Then, 24.4 g of 2-hydroxyethyl acrylate was added while controlling the temperature at 20-30° C. After reacting for a further one hour at 35° C., 420.61 g of a ring-opening copolymer of propylene oxide having a number average molecular weight of 2,000 (“ACCLAIM 2200” manufactured by Lyondell, unsaturated group content <0.01 meq / g) was added and the mixture was stirred at 50-60° C. for 5 hours. The reaction was terminated when the amount of the residual isocyanate was 0.1 wt % or less. 58.3 g of M-113, 139.9 g of isobornyl acrylate (manufactured by Osaka Organic Chemical Industry Co., Ltd.), 71.4 g of laury...
examples 6 and 7
[0085] A reaction vessel equipped with a stirrer was charged with 53.5 g of 2,4-tolylene diisocyanate, 150 g of nonylphenol EO-modified (4 mols) acrylate (monofunctional acrylate “Aronix M-113” manufactured by Toagosei Co., Ltd.), 0.1 g of 2,6-di-t-butyl-p-cresol, and 0.4 g of dibutyltin dilaurate. The mixture was cooled with ice to a temperature of 10° C. or less while stirring. Then, 24.0 g of 2-hydroxyethyl acrylate was added while controlling the temperature at 20-30° C. After reacting for a further one hour at 35° C., 203.9 g of a ring-opening copolymer of propylene oxide and ethylene oxide having a number average molecular weight of 2,000 (“ACCLAIM 2220” manufactured by Lyondell, copolymerization ratio, 90:10, unsaturated group content <0.01 meq / g) and 317.0 g of polypropylene glycol with a the number average molecular weight 3,000 (“EXENOL3000” made by Asahi Glass Co., Ltd.) were added and the mixture was stirred at 50-60° C. for 5 hours. The reaction was terminated when the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tg | aaaaa | aaaaa |
| Tg | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com