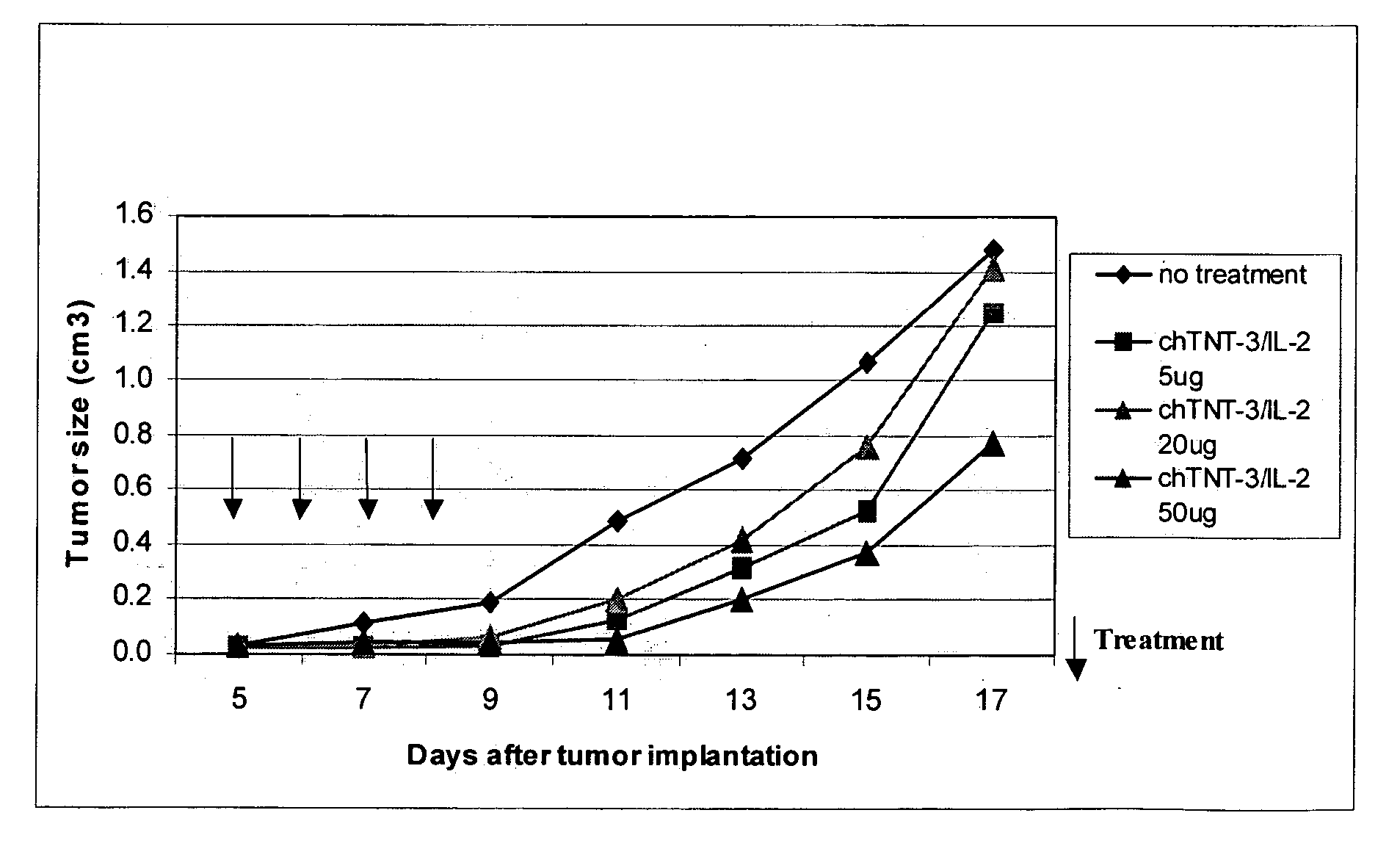
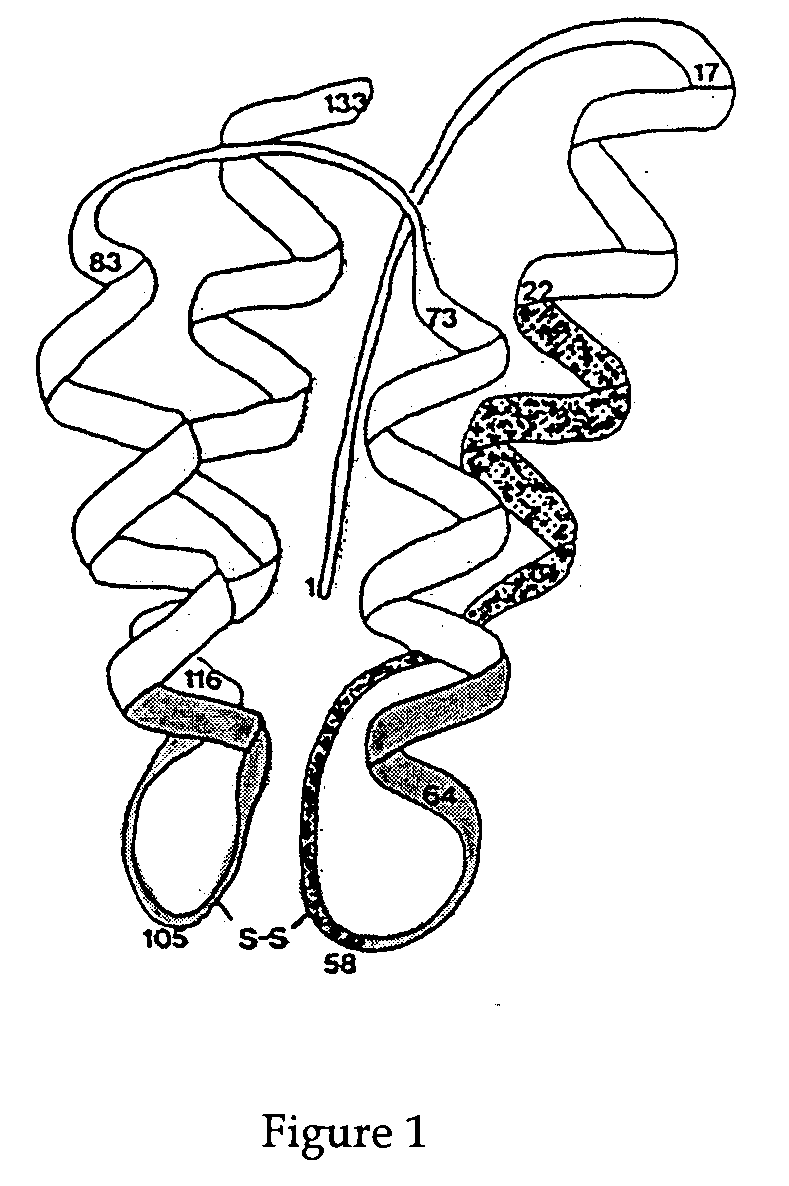
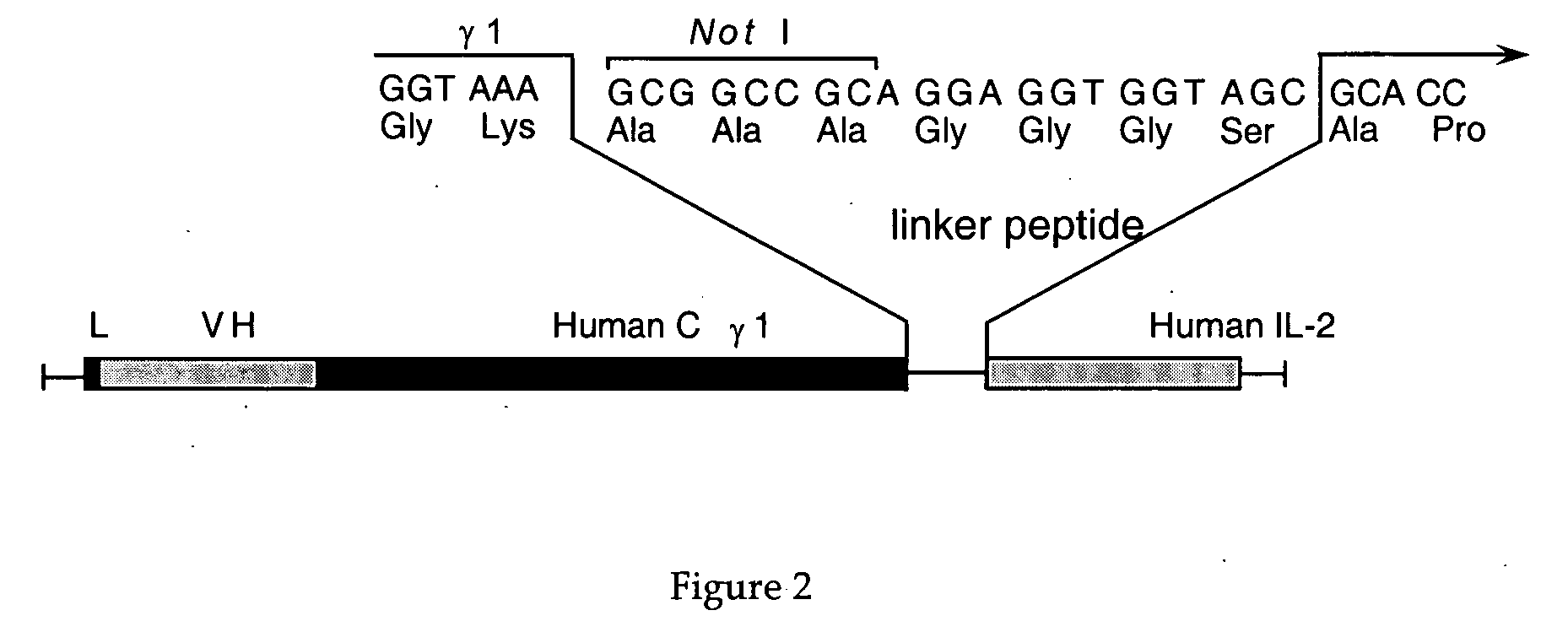
Interleukin-2 mutants with reduced toxicity
a technology of interleukin-2 and mutants, applied in the field of interleukin-2 (il -), can solve the problems of reduced toxicity of il-2, reduced vasopermeability activity, nausea and neuropathy, etc., and achieves similar binding affinity, reduced toxicity, and reduced vasopermeability activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Reagents
[0067] This example provides the preferred reagents for practice of the embodied invention. One skilled in the art can readily appreciate comparable materials that can be substituted in place of these reagents.
[0068] The Glutamine Synthase Gene Amplification System, including the expression plasmids pEE6 / hCMV-B and pEE 12 as well as the NSO murine myeloma expression cell line, were purchased from Lonza Biologics (Slough, UK). Restriction endonucleases, T4 DNA ligase, Vent polymerase, and other molecular biology reagents were purchased from either New England Biolabs (Beverly, Mass.) or Boehringer Mannheim (Indianapolis, Ind.). Dialysed fetal bovine serum, crude DNA from salmon testes, single-stranded DNA from calf thymus, chloramine T, and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt (ABTS) were purchased from Sigma Chemical Co. (St. Louis, Mo.). Recombinant human interleukin-2 was purchased from Chiron (Emeryville, Calif.). The Griess Reagent Syst...
example 2
Development and Characterization of IL-2 Mutant Polypeptides
[0070] This example provides methods of creating IL-2 mutant polypeptides and chimeric antibody / IL-2 fusion proteins (mutant or native). In addition, this example provides methods for determining the cytokine function and binding properties of resultant IL-2 molecules in vitro.
[0071] A. Construction and Expression of IL-2 and Antibody / IL-2 Fusion Proteins.
[0072] The construction of the chimeric monoclonal antibody TNT-3 (chTNT-3, IgG1, κ) and the fusion protein of this antibody with IL-2 have been previously described (Hornick et al., Cancer Biotherapy &Radiopharmaceuticals 13:255, 1998; Hornick et al., J. Nucl. Med. 41:355, 2000).
[0073] IL-2 mutant cDNA was prepared by site-directed mutagenesis to mutate amino acid 20 from aspartic acid to lysine (D20K), amino acid 38 from arginine to glycine (R38G) or tryptophan (R38W), amino acid 39 from methionine to valine (M39V) or leucine (M39L), amino acid 42 from phenylalanine ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| binding affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com