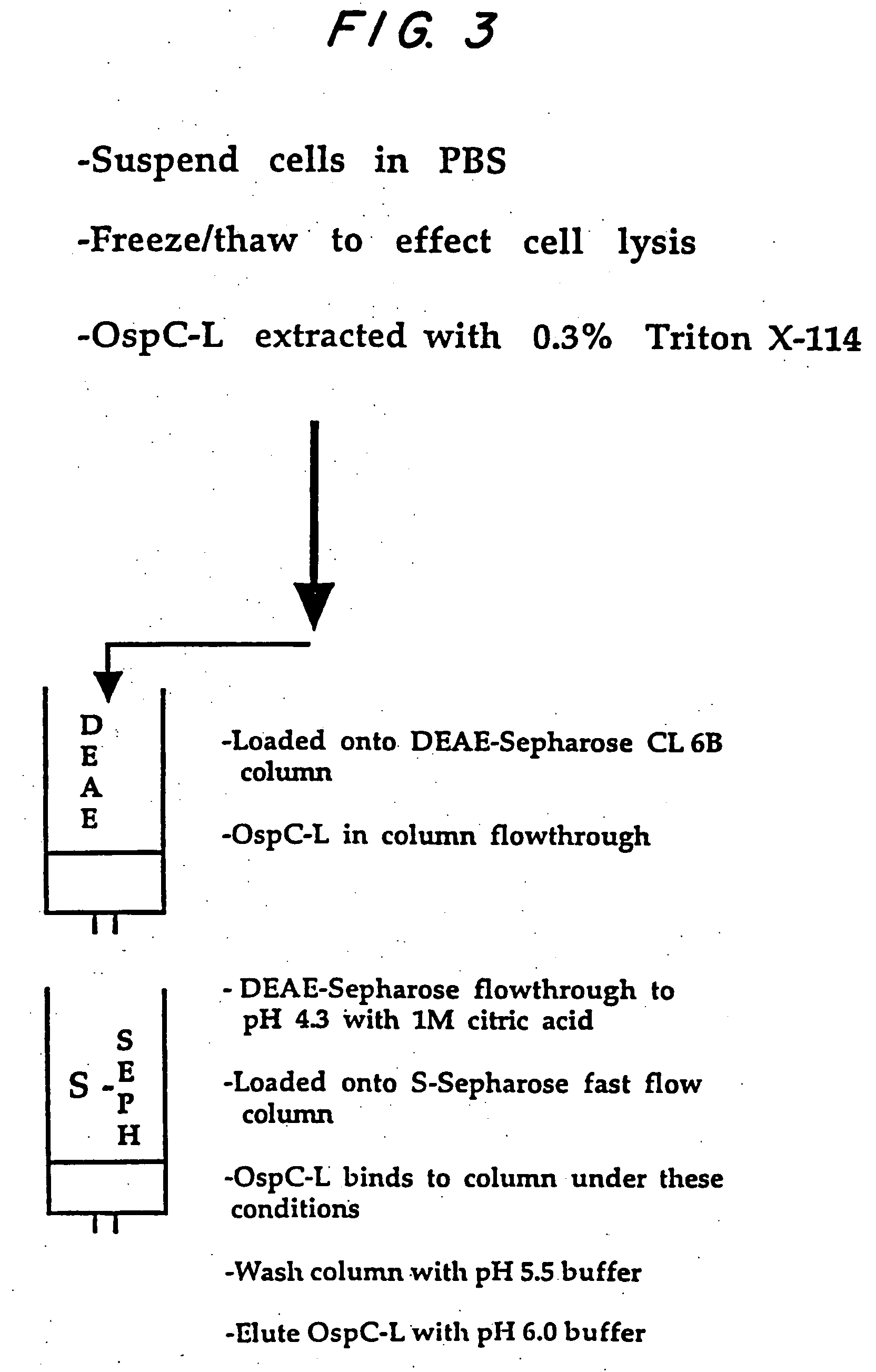
Expression of lipoproteins
a technology of lipoproteins and lipids, applied in the field of gene engineering, can solve the problems of presenting problems such as the problem of pneumococcal disease control, applicants' difficulty in obtaining detectable expression of recombinant ospc, and antibiotic resistance, and achieve the effect of reducing the risk of pneumococcal resistan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of a Vector Containing a Gene Encoding the OspA Leader Sequence
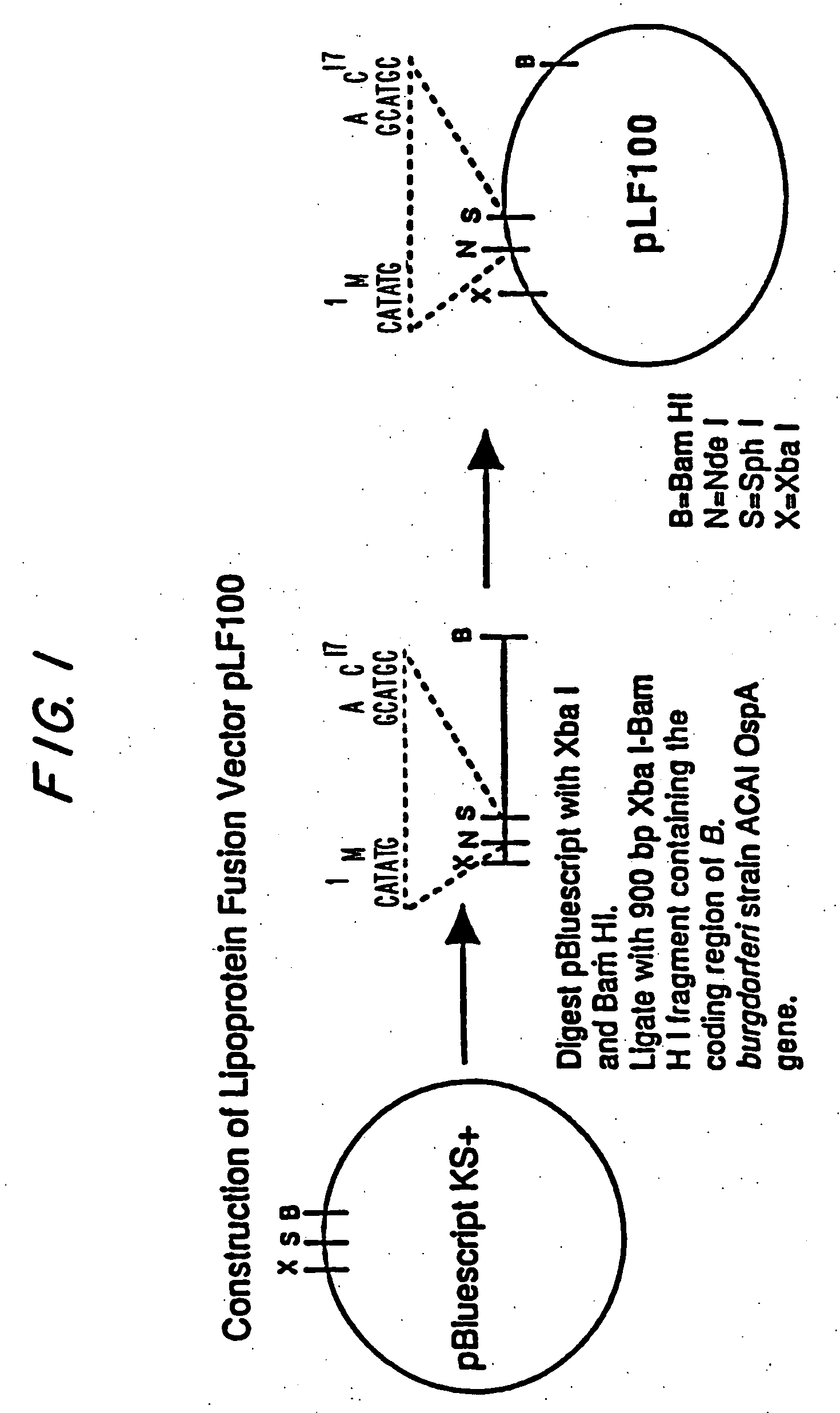
[0088] Plasmid pBluescript KS+ (Stratagene) was digested with XbaI and BamHI and ligated with a 900 bp XbaI-BamHI DNA fragment containing the complete coding region of B. burgdorferi strain ACA1 ospA gene, to form a lipoprotein fusion vector pLF100. This procedure is shown schematically in FIG. 1.
[0089] The vector pLF100 has been deposited with the American Type Culture Collection at Rockville, Md. on Feb. 2, 1995 under Accession No. 69750. This deposit was made under the terms of the Budapest Treaty.
example 2
construction of a pET9a Expression Vector Containing a Hybrid ospA-ospC Gene
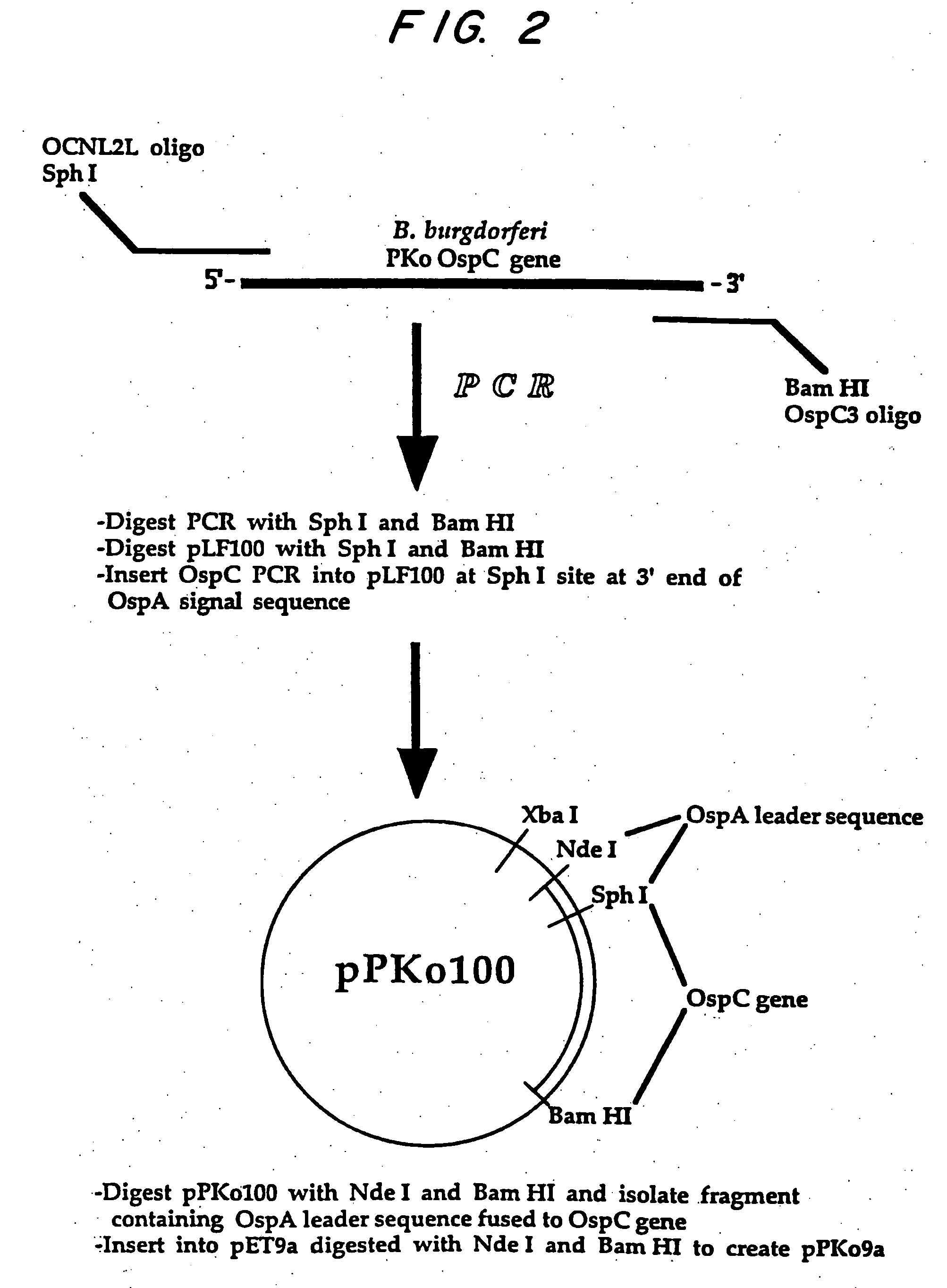
[0090] Specifically designed oligonucleotide primers were used in a polymerase chain reaction (PCR) to amplify the portion of the ospc gene downstream from the cysteine-encoding codon terminating the signal-peptide recognition-encoding sequence to the C-terminal end of the coding region from the Pko and B31 strains of B. burgdorferi.
[0091] The 5′-end primer had the nucleotide sequences respectively for the Pko and B31 strains:
5′-GGC GCG CAT GCA ATA ATT(Pko)(SEQ ID NO: 3)CAG GGA AAG G-3′5′-GGC GCG CAT GCA ATA ATT(B31)(SEQ ID NO: 4)CAG GGA AAG A-3′while the 3′-end primer had the nucleotidesequence:5′-CGC GGA TCC TTA AGG TTT(B31 &(SEQ ID NO: 5)TTT TGG-3′Pko)
[0092] The PCR amplification was effected in a DNA Thermal Cycler (Perkins-Elmer Cetus) for 25 cycles with denaturation for 30 secs at 94° C., annealing at 37° C. for 1 minute and extension at 72° C. for 1 minute. A final extension was effected at 72° C...
example 3
Expression and Purification of Lipidated OspC.
[0095] Plasmid pPko9a, prepared as described in Example 2, was used to transform E. coli strains BL21(DE3) (pLysS) and HMS174(DE3)(pLysS). The transformed E. coli was inoculated into LB media with 30 μg / ml kanamycin sulfate and 25 μg / ml of chloramphenicol at a rate of 12 ml of culture for every liter prepped. The culture was grown overnight in a flask shaker at 37° C.
[0096] The next morning, 10 ml of overnight culture medium was transferred to 1 L of LB media containing 30 μg / ml of kanamycin sulfate and the culture was grown in a flask shaker at about 37° C. to a level of OD600=0.6-1.0 (although growth up to OD600=1.5 can be effected), in approximately 3-5 hours.
[0097] To the culture medium was added isopropylthiogalactoside (IPTG) to a final concentration of 0.5 mM and the culture medium was grown for a further two hours at about 30° C. The cultures were harvested and samples analyzed on Coomassie stained SDS-PAGE gels (FIG. 5). The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com