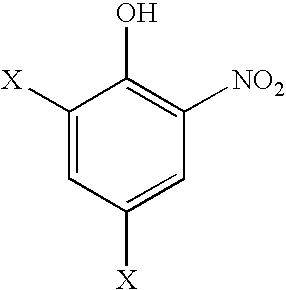
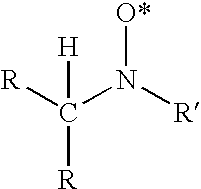
Composition and method for inhibiting polymerization and polymer growth
a technology polymerization inhibition, which is applied in the direction of thermal non-catalytic cracking, separation processes, fuels, etc., can solve the problems of loss of monomers, reduced yield, undetectable polymerization of ethylenically unsaturated monomers, etc., to prevent polymer growth, the effect of preserving and utilizing the effectiveness of nitroxyl radical inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Procedure for Polymer Growth Reboiler Test Preparation of Feed Solution
[0175] Tert-butylcatechol (TBC) is removed from commercially available styrene by distillation under vacuum. Removal of TBC is verified by caustic titration. The desired amount of inhibitor(s) is added to this TBC-free styrene either directly or by first making a concentrated solution of the inhibitor in TBC-free styrene followed by further dilution with TBC-free styrene.
Procedure for Polymer Growth Dynamic Reboiler Test:
[0176] A quantity of the Feed Solution containing inhibitor or blend of inhibitors at the desired charge (stated as a wt / wt total inhibitor to styrene) is added to a round-bottom flask (the Pot). A known quantity of insoluble polymer capable of growing via a living mechanism is placed inside the Pot and submersed in the Feed Solution in the Pot. The insoluble polymer can be retained in the Pot by any suitable means. Typically, the insoluble polymer is securely wrapped in a piece of filter pape...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com