Novel alcohol/aldehyde dehydrogenases
a technology of alcohol/aldehyde dehydrogenase and recombinant enzymes, which is applied in the field of recombinant enzymes, can solve the problem of no reports of cloning of such genes so far
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning of AADH Genes
(1) Construction of a Genomic Library of G. oxydans DSM No. 4025
[0104] Chromosomal DNA was prepared as follows. G. oxydans DSM No. 4025 was cultivated on an agar plate containing 20 ml of NS2 medium consisting of 5.0% D-mannitol, 0.25% MgSO4.7H2O, 1.75% corn steep liquor, 5.0% baker's yeast (Oriental Yeast Co., Osaka, Japan), 0.5% CaCO3, 0.5% urea (sterilized separately) and 2.0% agar (pH 7.0 before sterilization) at 27° C. for 3 days. The cells were collected from the agar plate, washed with 10 ml of 10 mM Tris-HCl buffer (pH 8.0) containing 1 mM EDTA and resuspended in 5 ml of 10 mM Tris-HCl buffer (pH 8.0) containing 20 mM EDTA. The cell suspension was treated with lysozyme (Sigma Chemicals Co., St. Louis, Mo., USA) at a final concentration of 400 μg / ml at 37° C. for 30 minutes, then with pronase (400 units) at 37° C. 30 minutes and with 1% SDS at 37° C. for 1 hour. Chromosomal DNA was treated with phenol and RNase A (Boheringer Mannheim, GmbH, Mannheim, G...
example 2
Nucleotide Sequencing
[0112] Nucleotide sequences of the genes for Enzymes A, A′, A″ and B were determined with the plasmids, p24D4, p1E2, p26C3, and pSS31, respectively, by the dideoxynucleotide chain termination method using M13mp18 and M13mp19 (Boehringer Mannheim). One open reading frame (ORF) for each gene was found; the nucleotide sequences of the four genes are shown in the sequence list SEQ ID NOS. 1 to 4 and the amino acid sequences deduced from the nucleotide sequences were shown in the sequence list SEQ ID NOS. 5 to 8. The ORFs for Enzymes A, A′, A″ and B genes are 1737, 1737, 1734, and 1737-bp long and encode 579, 579, 578 and 579 amino acid residues all including 23 amino acid of signal sequences.
[0113] The homologies between Enzymes A, A′, A″ and B are shown in Table 7.
TABLE 7Homologies of amino acid sequences among AADHs.(%)Enzyme AEnzyme A′Enzyme A″Enzyme BEnzyme A100———Enzyme A′89100——Enzyme A″8586100—Enzyme B838281100
FIG. 5 shows the amino acid sequences of matu...
example 3
Subcloning of AADH Genes
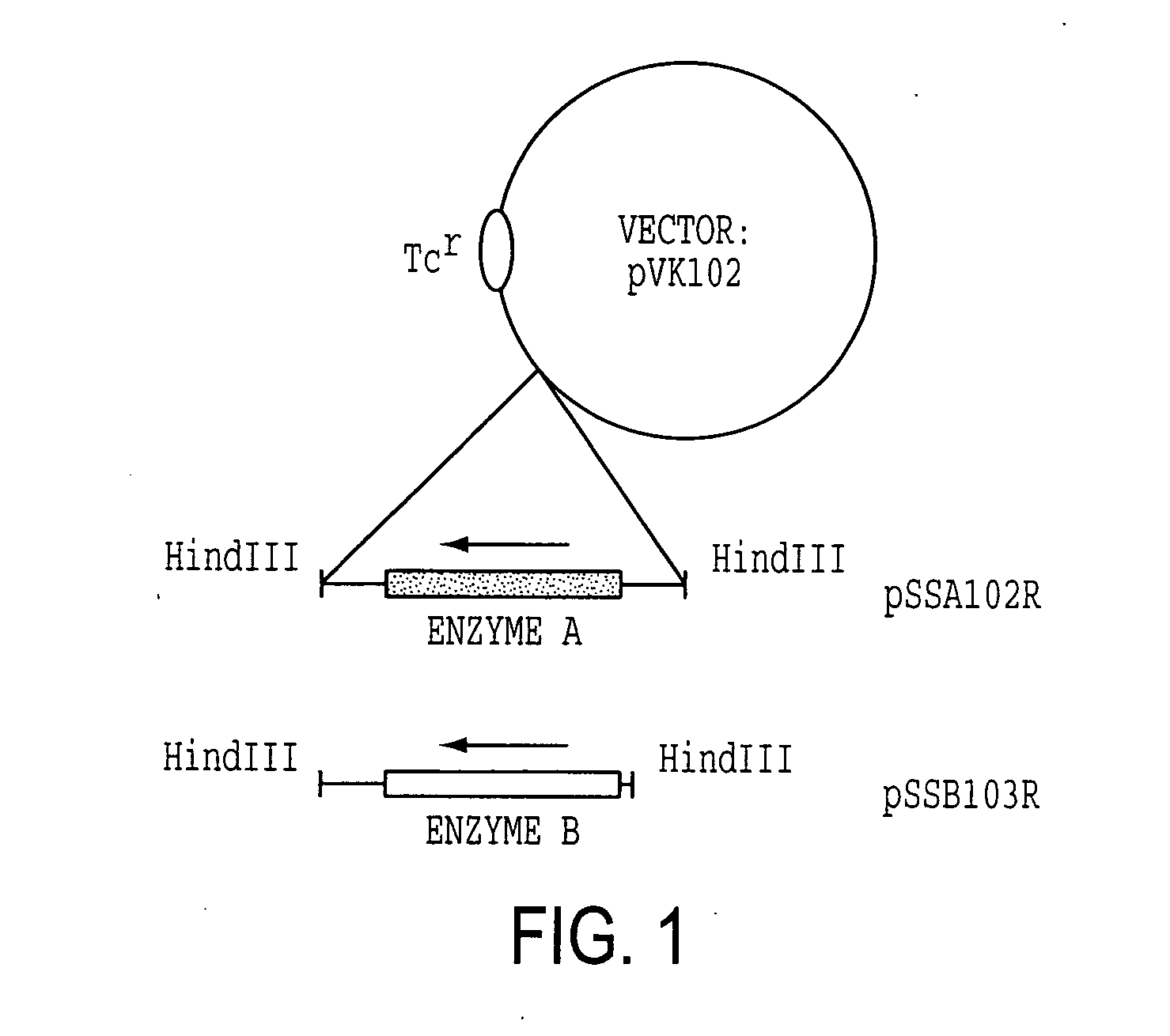
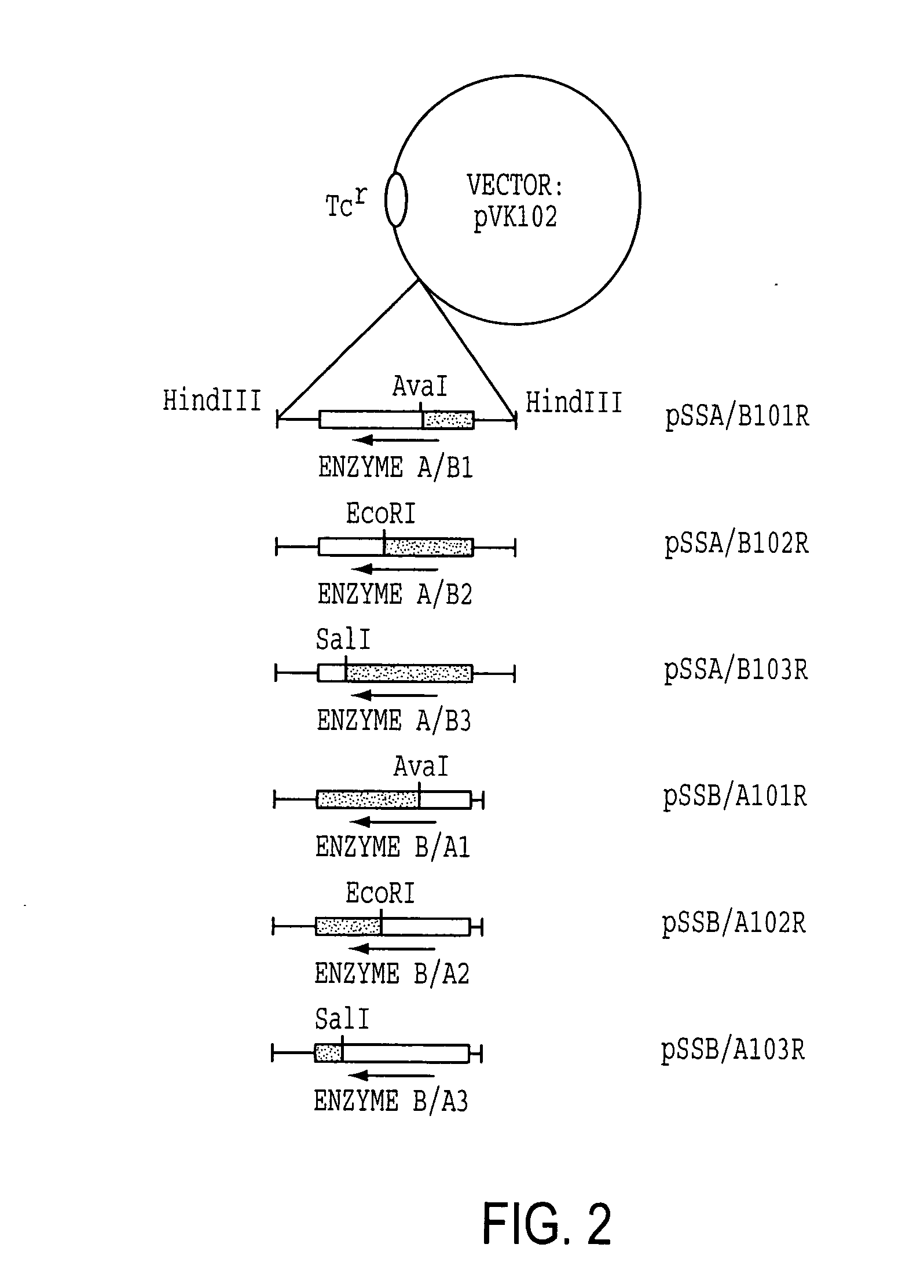
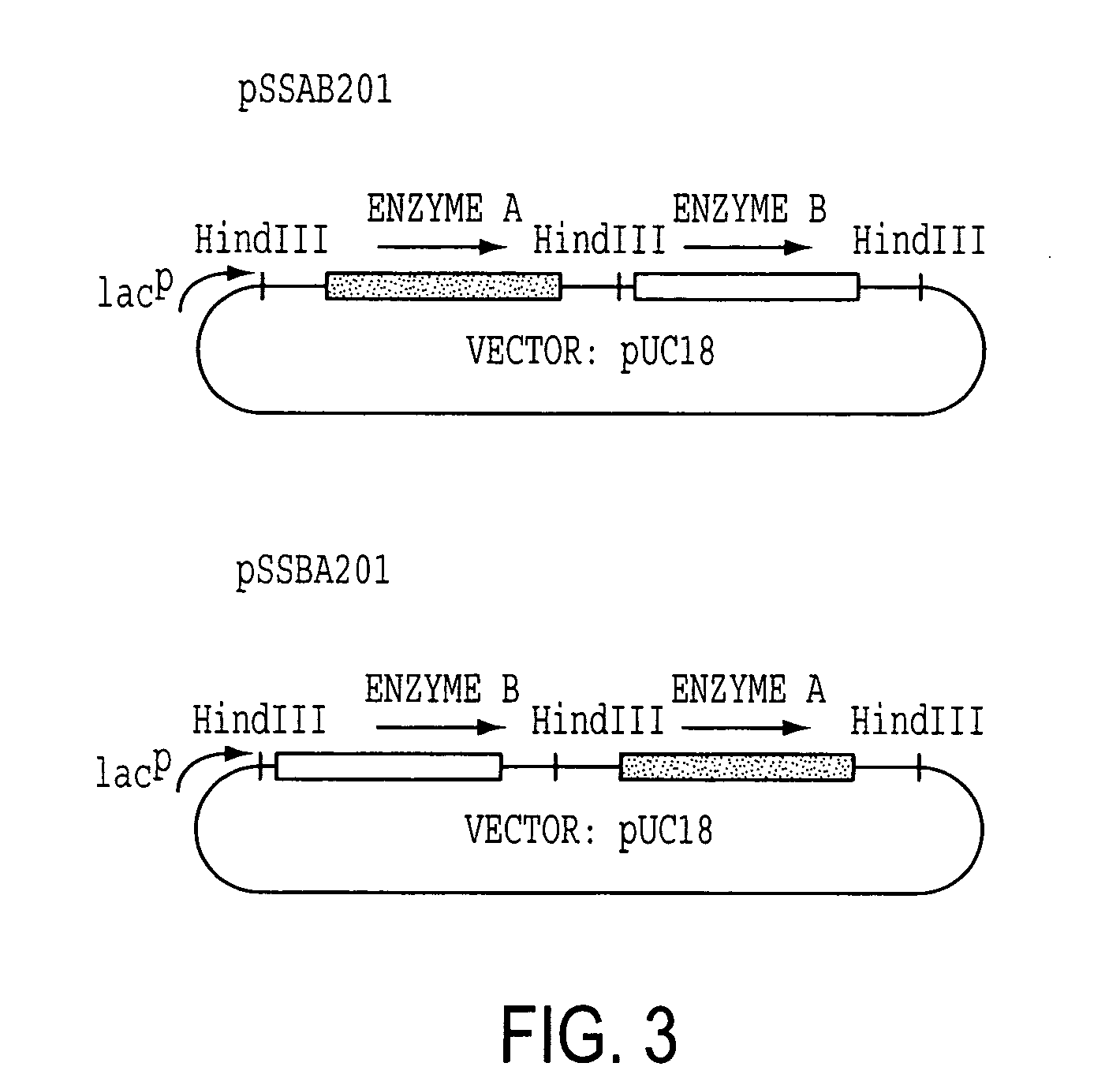
[0114] Enzyme A gene was originally cloned as a cosmid clone of p24D4 which has about 25 kb insert in EcoRI site of pVK100. Then, it was further subcloned to use as an Enzyme A gene cassette. The 2.7 kb EcoRV fragment which includes ORF of Enzyme A gene with about 500 bp of non-coding regions at the both ends was excised from 3.4 kb NruI fragment, which was isolated from p24D4 in M13 mp18, and was ligated to HindIII site of pUC18 with HindIII linker (CAAGCTTG). The resulting plasmid was designated pSSA202. Enzyme A gene cassette (2.7 kb HindIII fragment) was then inserted at HindIII site of pVK102 to produce pSSA102R. The plasmid pSSA102R was introduced into nalidixic acid resistant P. putida [ATCC 21812] by a conjugal mating method as described in Example 1-(1). The transconjugant of P. putida carrying pSSA102R was selected on MB agar medium containing 50 μg / ml nalidixic acid and 10 μg / ml tetracycline (MNT agar medium) and subjected to a mini-resting cell r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com