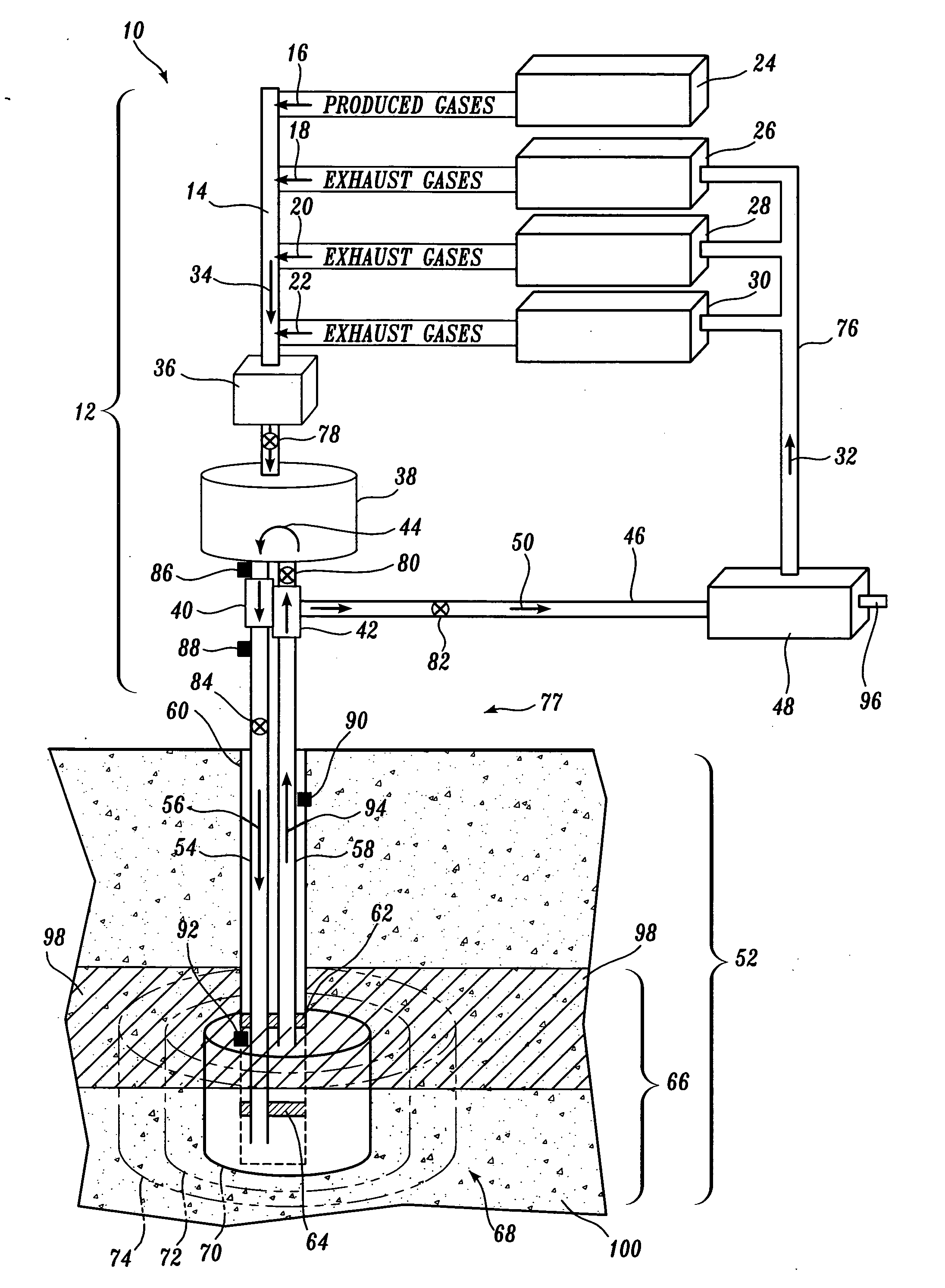
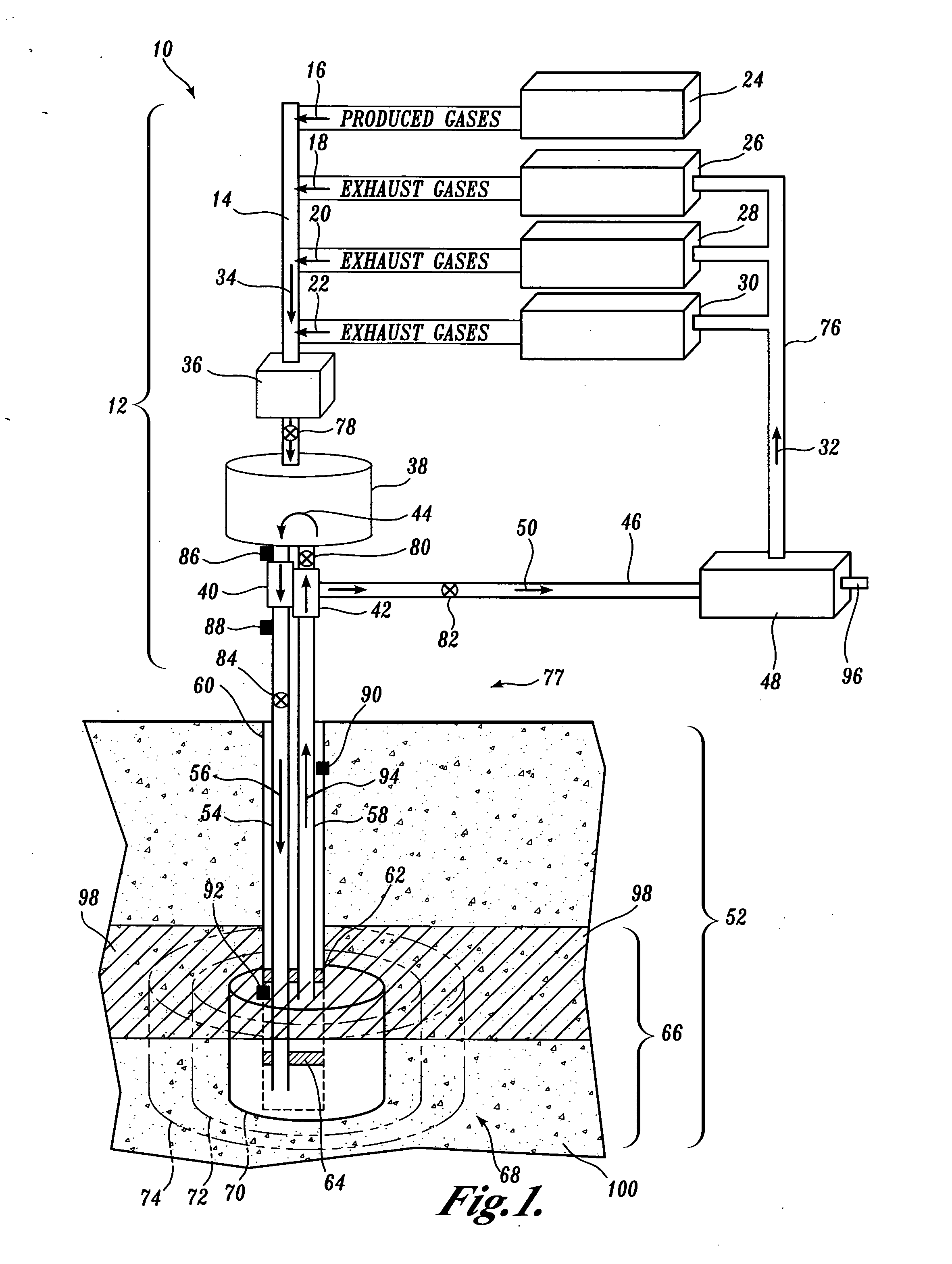
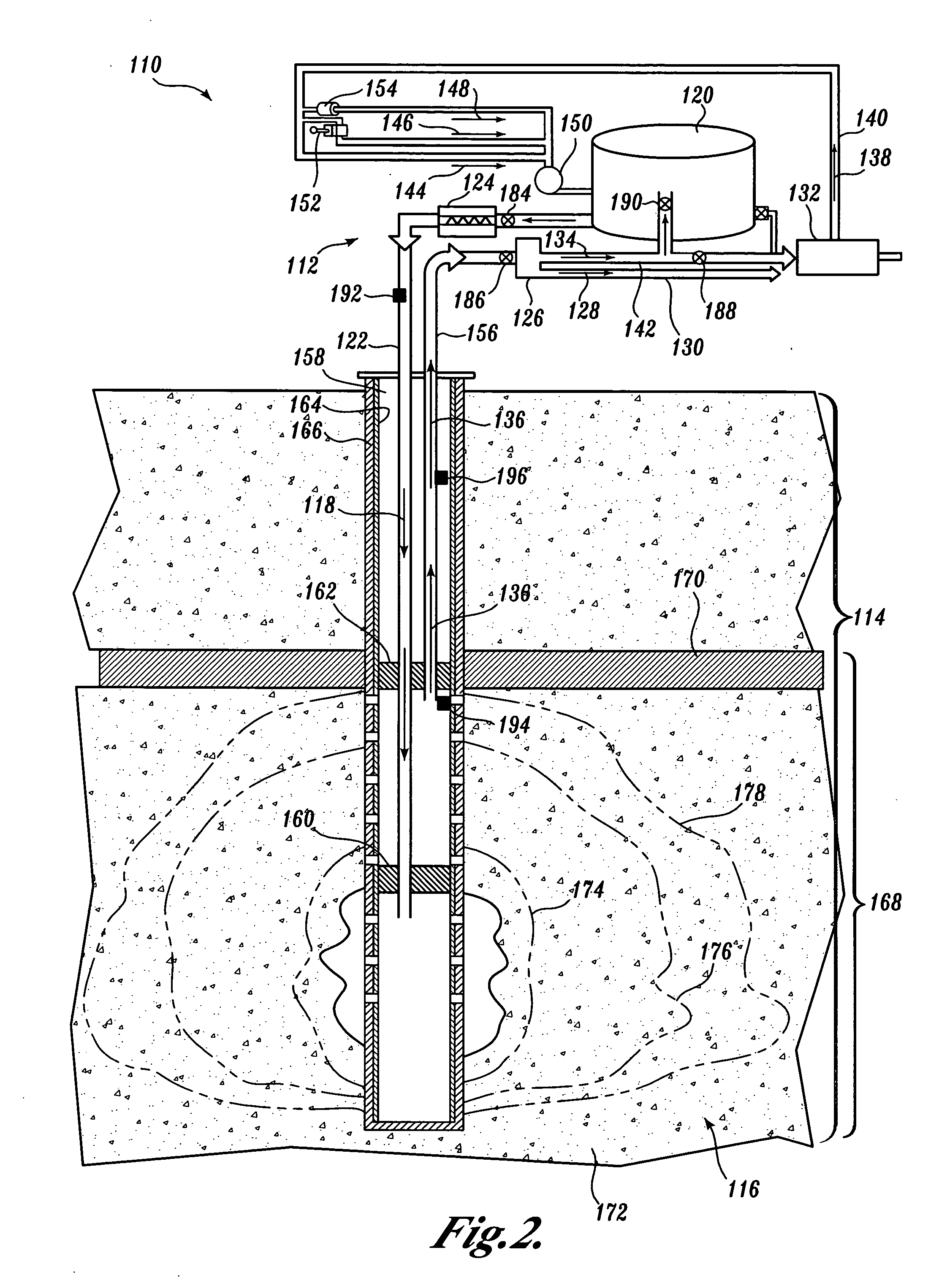
However, no
active method for the sequestration of carbon dioxide into
hydrate bearing subterranean zones exists.
The additional capital, fuel, operations, and maintenance costs of CO.sub.2 capture add 1 to 3 cents per kWhr to the
electricity cost of base plants.
Part of the cost of CO.sub.2 capture is due to energy requirements of the capture process that result in net power losses.
Other than the experiences discussed above, there are no large scale commercial carbon capture and sequestration technologies currently deployed."
Unless there are carbon control policies like the Norwegian carbon tax, such high CO.sub.2 capture and sequestration costs are not likely to provide incentives for the deployment of these technologies."
However, no active production technique exists for the production of hydrocarbons from hydrates in subterranean zones.
Despite all of these mature technologies, the art of sequestering carbon dioxide and the art of producing hydrates have not been undertaken jointly.
This in turn leads to increase in size and cost of the equipment in the
plant utilizing the natural gas and in the cost of development of the pipeline.
These current costs of carbon dioxide capture add 1 to 3 cents per kWhr to the
electricity cost of base plants.
However, storage of the carbon dioxide for long periods of time has not been evaluated.
(a) The liquid and
vapor phase of the injectant co-exist and the injectant has a density greater than the vapor released upon dissociation of the
hydrate (areas 300 and 504);
(b) The
liquid phase of the injectant exists (that is, there is no
vapor phase of the injectant) or the
critical phase of the injectant exists and the injectant has a density greater than the vapor released upon dissociation of the
hydrate and the pressure is below the
fracture pressure gradient (areas 252, 302, 402, 452, and 502); and
(c) The pressure is above the
fracture pressure gradient (areas 250, 304, 348, 400, 450, and 500).
2. The phase envelope (lines 258, 310, 358, 410, 458, and 510) for 100%
methane. For this phase envelope, the hydrate phase is in the higher pressure and lower temperature region and the
vapor phase is in either the lower pressure or higher temperature regions. The typical composition of an in-place hydrate is near 100%
methane.
3. The specific pressure and temperature profile of a well (lines 256, 308, 356, 406, 456, and 508) within the area of hydrate occurrence. This is the pressure and temperature profile for each well. The intersection of the well bore pressure and
temperature gradient (lines 256, 308, 356, 406, 456, and 508) with the methane phase envelope (lines 258, 310, 358, 410, 458, and 512) shows where the in-place hydrates are stable and can exist in the subsurface location.
4. The fracture gradient for the well (lines 254, 306, 354, 404, 454, and 506) is the
injection pressure which, if exceeded, will fracture the formation. The
fracture pressure of a well, where hydrates are stable, depends upon the depth of the hydrates. The maximum fracture pressure is based upon the
maximum depth of the hydrate occurrence. Fractures can breach the subsurface containment and cause pressure leakoff. Vertical fractures can leak off to low pressure zones. Whether vertical fractures occur is dependent on local
stress conditions or perhaps other unknown factors. Horizontal fractures may even help the production process.
5. The ocean floor (lines 412, 462, and 514).
A
vertical fracture that fractures the overlying shale may be a problem because of the migration of gas along that fracture and potential subsequent breach of containment and depressurization of injectant.
The cost of removing NO.sub.x from
flue gas drives up the cost of production and may make the process too expensive.
However no matter what the type of fracture, vertical or horizontal (or parallel to the
bedding), the injectant should not be injected at a high enough pressure to cause the subsurface containment
system to be breached by a fracture that would cause insufficient pressure retention in the subsurface containment subsystem for the injectant to exist as a
liquid phase.
Emplacement of the injectant in this zone may not be possible because dynamic subsurface containment cannot be maintained.
Increasing the pressure in this zone is dependent on the size of the
aquifer underlying the
free gas zone and chances are that the zone will be limited to
pressure increase.
A
vertical fracture that fractures the overlying shale or subsurface containment may be a problem because of the migration of gas along that fracture and potential subsequent breach of containment and depressurization of injectant.
However no matter what the type of fracture, vertical or horizontal, the injectant should not be injected at a high enough pressure to cause the subsurface containment
system to be breached by a fracture that would cause insufficient pressure retention in the subsurface containment subsystem for the injectant to exist as a
liquid phase.
Because of the 8:1 ratio of water to gas in the melting of the hydrate, there is expected to be significant retardation of mixing the hydrate-evolved natural gas and the injectant resulting in only minor mixing of the evolved natural gas with the injectant.
Maintenance of a higher pressure than the original pressure in the
free gas zone may not be possible, so maintaining a pressure sufficient for the stability of the liquid injectant may be difficult in the
free gas zone.
Higher pressure and temperature for the injectant increase the costs of the injectant.
 Login to View More
Login to View More  Login to View More
Login to View More