Support for analyte determination methods and method for producing the support
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
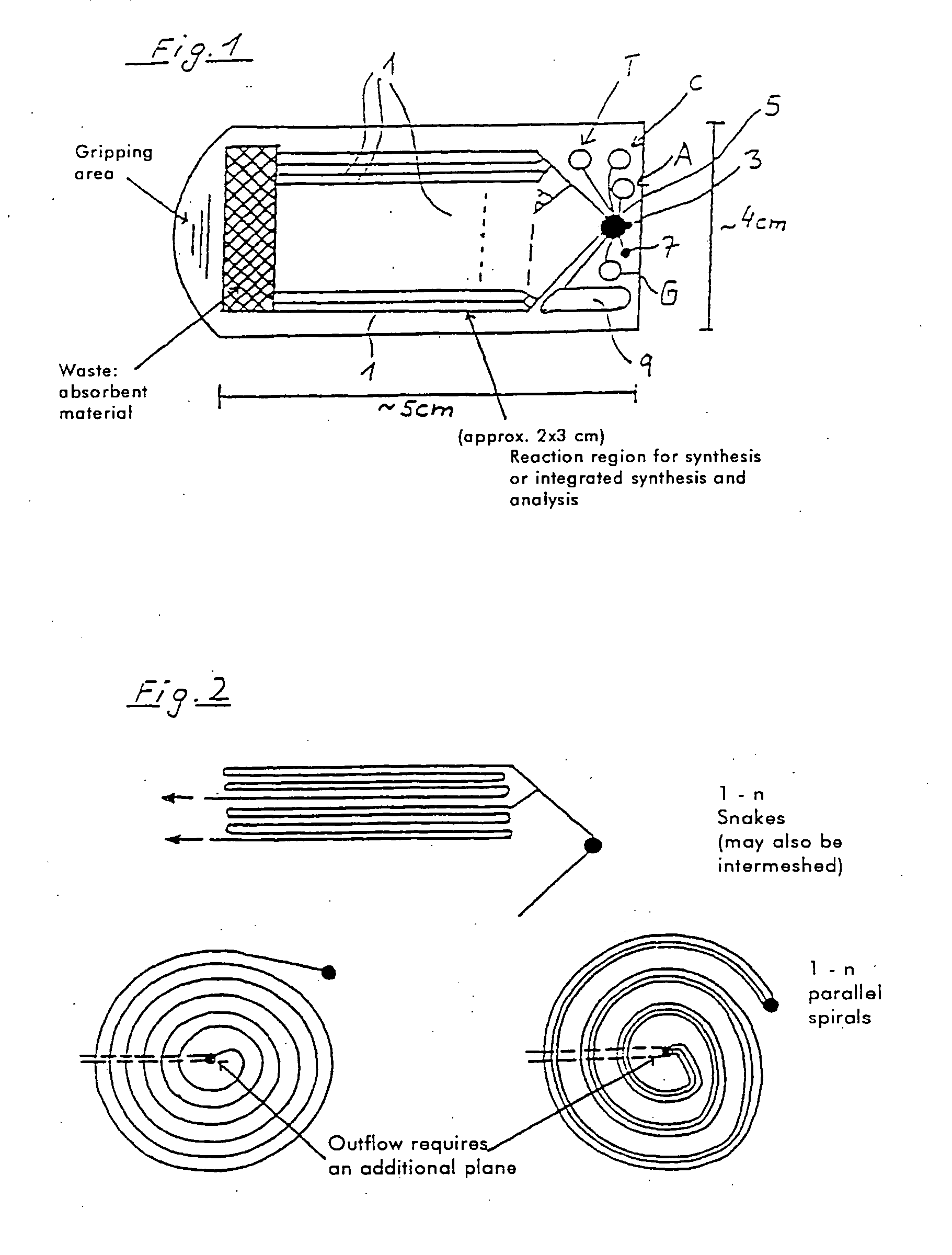
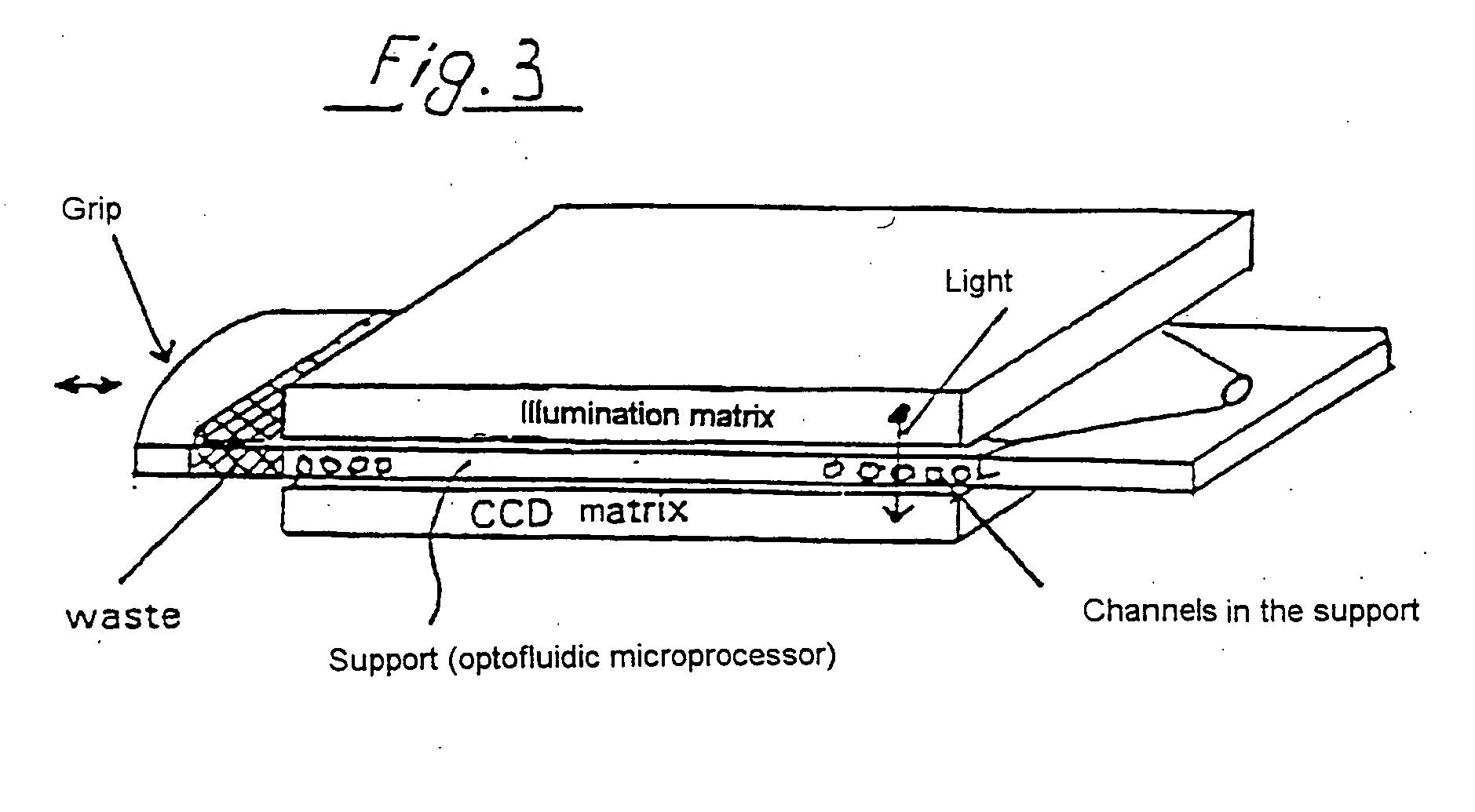
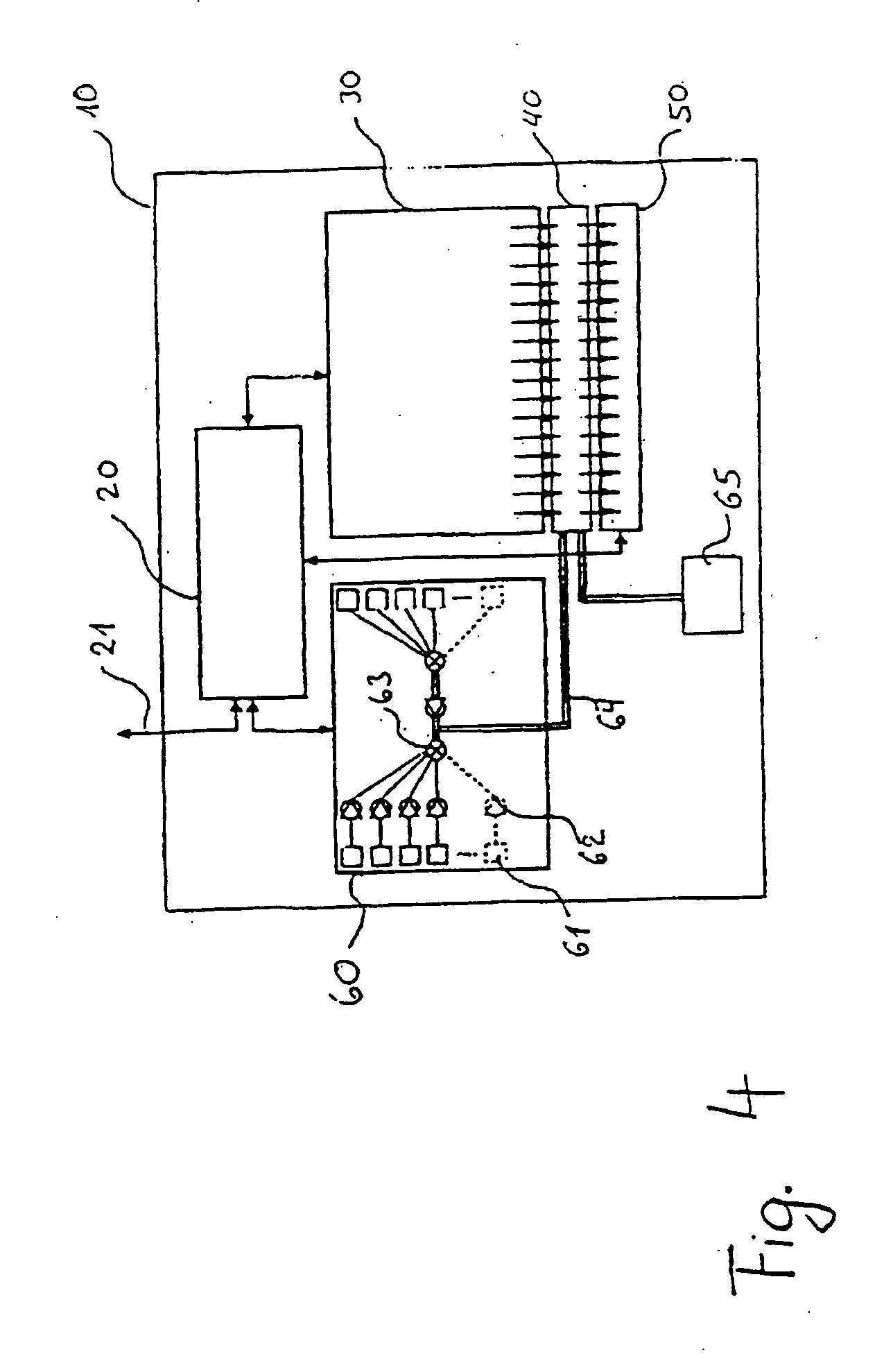
[0186] The synthesis of DNA molecules in channels can take place with use of standard synthons, for example phosphoramidite building blocks, with suitable protective groups, for example dimethoxytrityl (DMT). A corresponding fluidic DNA synthesis can take place starting from a linker coupled to the solid phase.
[0187] This format can be combined for the preferred embodiment of the invention with a light-dependent control of the DNA synthesis. For this purpose, protective groups which permit light-dependent deprotection are known, so that the protective group, which is usually linked on the 5' carbon atom of the synthon, is eliminated by light of suitable wavelength. The synthesis of nucleic acids with a length of 18 or more nucleotides is possible in capillaries in this way.
[0188] The reaction products can be analyzed, for example by high performance liquid chromatography (HPLC), by detaching the synthesized DNA oligomer, as is possible on use of suitable linkers. In this case it is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Area | aaaaa | aaaaa |
| Transparency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com