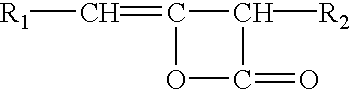
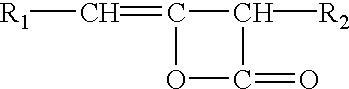
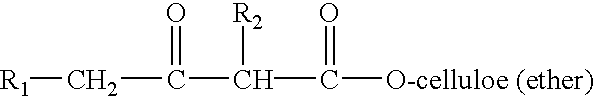
Method for the preparation of modified cellulose ethers
a technology of hydrophobic modification and cellulose ether, which is applied in the field of hydrophobic modification cellulose ether preparation, can solve the problems of long reaction time of manufacturing process, long reaction time of epoxy compound, hazardous to environment and health, etc., and achieves the effect of simple and quick
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0030] 100 g of CMC (Metsa Speciality Chemicals) having an average molecular weight of 180 000 and a degree of substitution of 0.65 was put into a steel container. 1 g of alkylketene dimer dispersion having an AKD content of 10% by weight, diluted with 50 g of water, was sprayed on CMC, agitating the powder at the same time. The product was dried at 80.degree. C. to a moisture content of less than 8%. The AKD content of the end product was 0.1% by weight.
examples 2-6
[0031] By using the procedure described above, the following hydrophobically modified CMC samples (examples 2-6) were prepared:
1 TABLE 1 Molecular Degree of AKD content weight substitution in CMC, Example of CMC of CMC % by weight 2 60 000 0.72 0.05 3 300 000 0.80 0.05 4 220 000 0.87 0.1 5 305 000 1.15 1.0 6 80 000 0.75 0.01 Sample 5 contained 0.02% by weight of AKD which had reacted with CMC.
example 7
[0032] 100 g of CMC having an average molecular weight of 40 000 and a degree of substitution of 0.79 was put into a steel cylinder. 5 g of solid alkylketene dimer was added. The powder was agitated in an oven at 60.degree. C. for 120 minutes and at 105.degree. C. for 15 min. The AKD content of the end product was 5.0% by weight.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com