Colorless petroleum marker dyes
a petroleum marker and dye technology, applied in the direction of diaryl/triaryl methane dyes, thickeners, fuels, etc., can solve the problems of phthalein derivatives and their typically low solubility in petroleum products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Di-n-Butyl Ester of o-Cresolphthalein
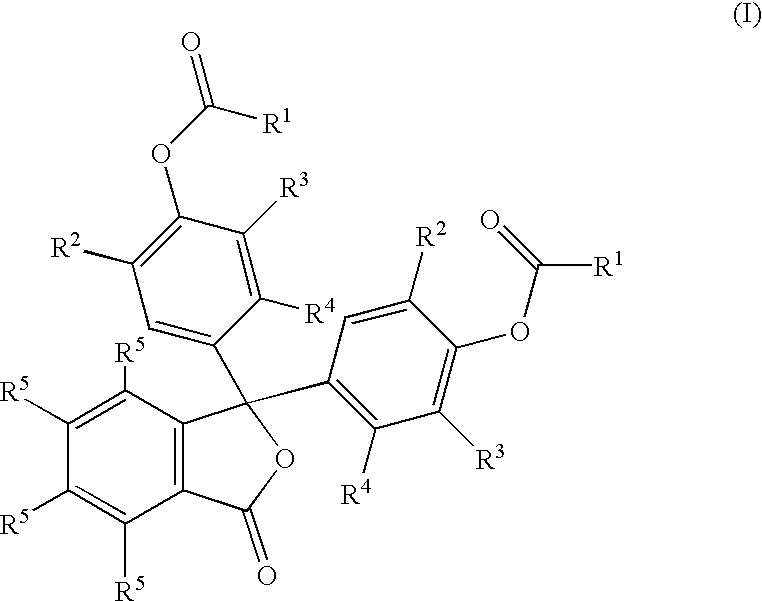
[0013] To a 100 mL, three-neck round-bottom flask fitted with a stirrer, thermocouple and a condenser, the following raw materials were added: (1) o-cresolphthalein, 1.73 g (0.005 moles); (2) water, 30 mL; (3) 50% NaOH, 0.8 g; (4) Na.sub.2CO.sub.3, 0.6 g; and (5) xylenes, 8 g. The mixture was stirred at 20-25.degree. C. for one hour, during which butyric anhydride, 2 g, was added. The mixture was then heated to 50-55.degree. C. until TLC revealed that the esterification reaction was complete. The upper xylenes layer was separated and 1-octyl-2-pyrrolidone, 2.5 g was added to it. Xylenes and water were removed azeotropically under vacuum. A residue of 6.6 g was obtained.
[0014] A marker solution was prepared by dissolving a 100 mg portion of the residue in xylenes (100 mL), and diluting 1 mL of the resulting solution to 100 mL with kerosene to make a 10 ppm solution.
[0015] A developer was made by dissolving 1 g of a 40% solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| color | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com