Resolution method for axis chiral enantiomers of lesinurad
a chiral enantiomer and axial enantiomer technology, applied in the field of pharmaceuticals, can solve the problems of low total yield, low optical purity, and inability to industrialize the resolution method by using instruments, and achieve the effects of low cost, low cost, and simple filtration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0079]Racemic lesinurad (2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid) was synthesized according to the method described in patent documents WO2009070740A2, CN103524440A or WO2014008295A1. The specific synthetic route is as follows:
[0080]
4-(1-cyclopropylnaphthalen-4-yl)-4H-1,2,4-triazol-thiol (compound A, 14.5 g, 54.2 mmol) was dissolved in 150 ml DMF, potassium carbonate (11.2 g, 81.3 mmol) was added to the solution, and then methyl bromoacetate (5.88 g, 54.2 mmol) was added dropwise. After the addition was complete, the solution was raised to 50° C. and kept for 4 hours. After the reaction was complete, the reaction solution was partitioned between water (100 ml) and ethyl acetate (100 ml). The aqueous phase was extracted with ethyl acetate (2×100 ml). The organic phases were combined, washed with saline (6×150 ml), dried over anhydrous sodium sulfate for 1 h, and filtrated with suction. The filtrate was evaporated to dryness to obtain 15.6 g w...
example 2
[0083]
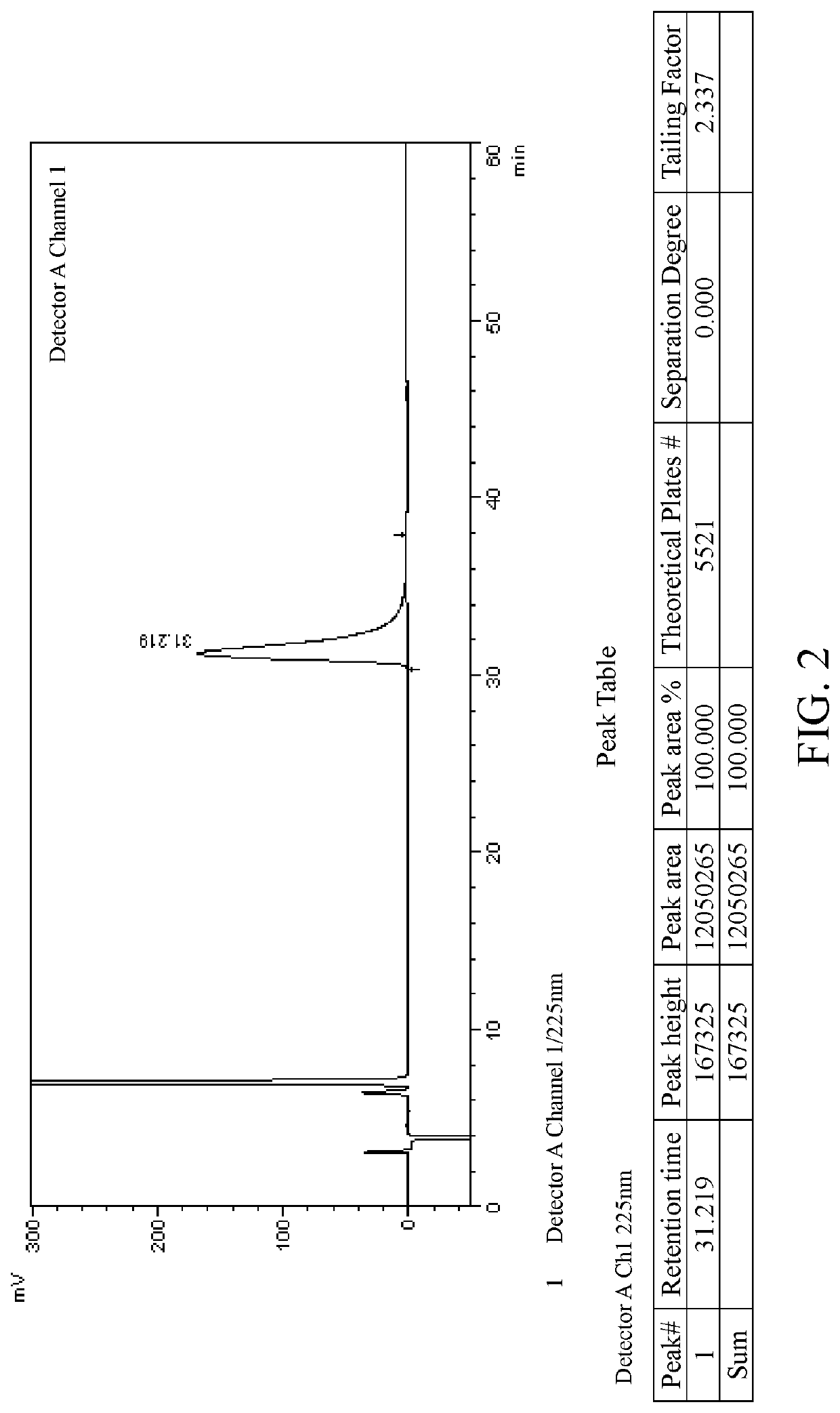
[0084]Salt formation: 10 g of lesinurad racemate (1.0 eq.) was added to ethyl acetate (100 ml). The mixture was heated to 70° C., and quinine (8.1 g, 1.0 eq.) was slowly added under stirring. The mixture was heated to reflux, and kept at the constant temperature for 1 h. The reaction system was slowly and naturally cooled down to room temperature, then kept in an ice-water bath and stirred for 30 mins. After filtration, the filter cake was washed with ethyl acetate (20 ml) to obtain 9.1 g white solid powder A (salt of lesinurad in R configuration), ee=97.9%. The filtrate was concentrated by rotary evaporation and dried to obtain 9.0 g white solid powder C (salt of lesinurad in S configuration), ee=94.5%.
[0085]Recrystallization: 9.1 g of the above white solid powder A was added to ethyl acetate (90 ml) under stirring. The mixture was heated to reflux, and kept at the constant temperature for 1 h. The reaction system was slowly and naturally cooled down to room temperature, then...
example 3
[0088]
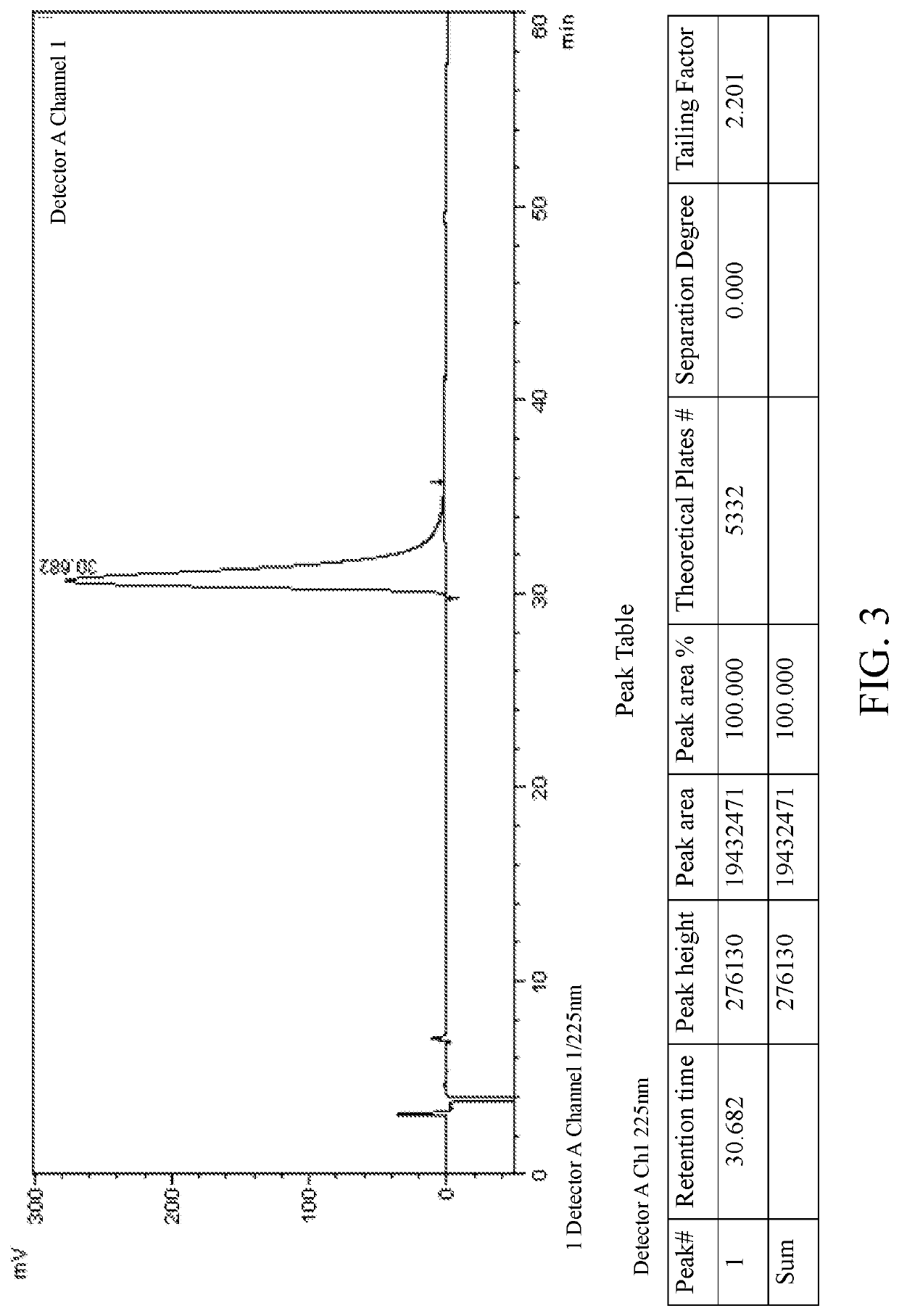
[0089]Salt formation: 10 g of lesinurad racemate (1.0 eq.) was added to toluene (100 ml). The mixture was heated to 105° C., and cinchonine (7.3 g, 1.0 eq.) was slowly added under stirring. The mixture was heated to reflux, and kept at the constant temperature for 1 h. The reaction system was slowly and naturally cooled down to room temperature, then kept in an ice-water bath and stirred for 30 mins.
[0090]After filtration, the filter cake was washed with toluene (20 ml) to obtain 8.9 g white-like solid (salt of lesinurad in S configuration, a crude product), ee=80%. The filtrate was concentrated by rotary evaporation and dried to obtain 9.1 g white solid powder (salt of lesinurad in R configuration, a crude product), ee=40%.
[0091]Recrystallization: 8.9 g of the above white-like solid (salt of lesinurad in S configuration, a crude product) was added to toluene (90 ml) under stirring. The mixture was heated to reflux, and then kept at the constant temperature for 1 h. The reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| chiral purity ee | aaaaa | aaaaa |
| chiral purity ee | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com