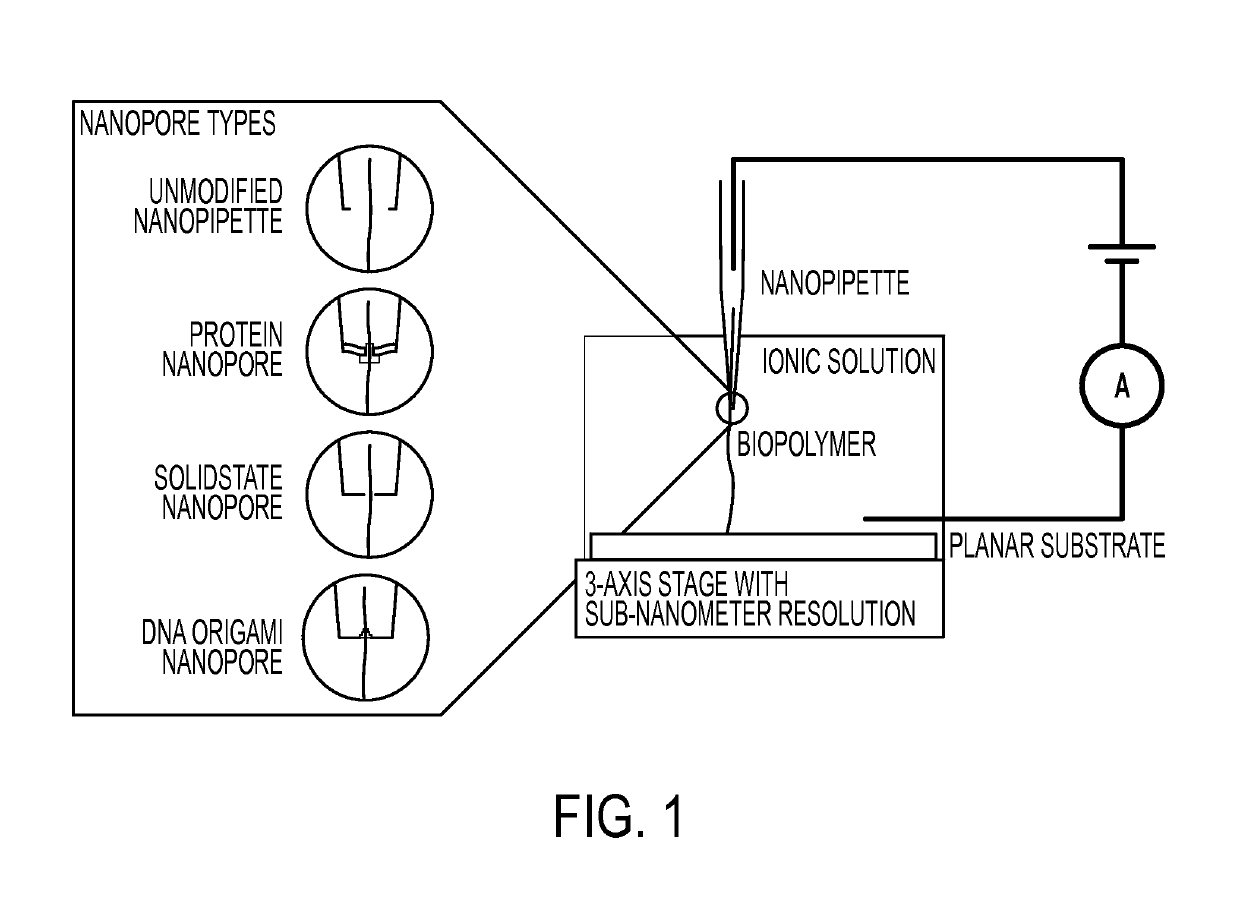
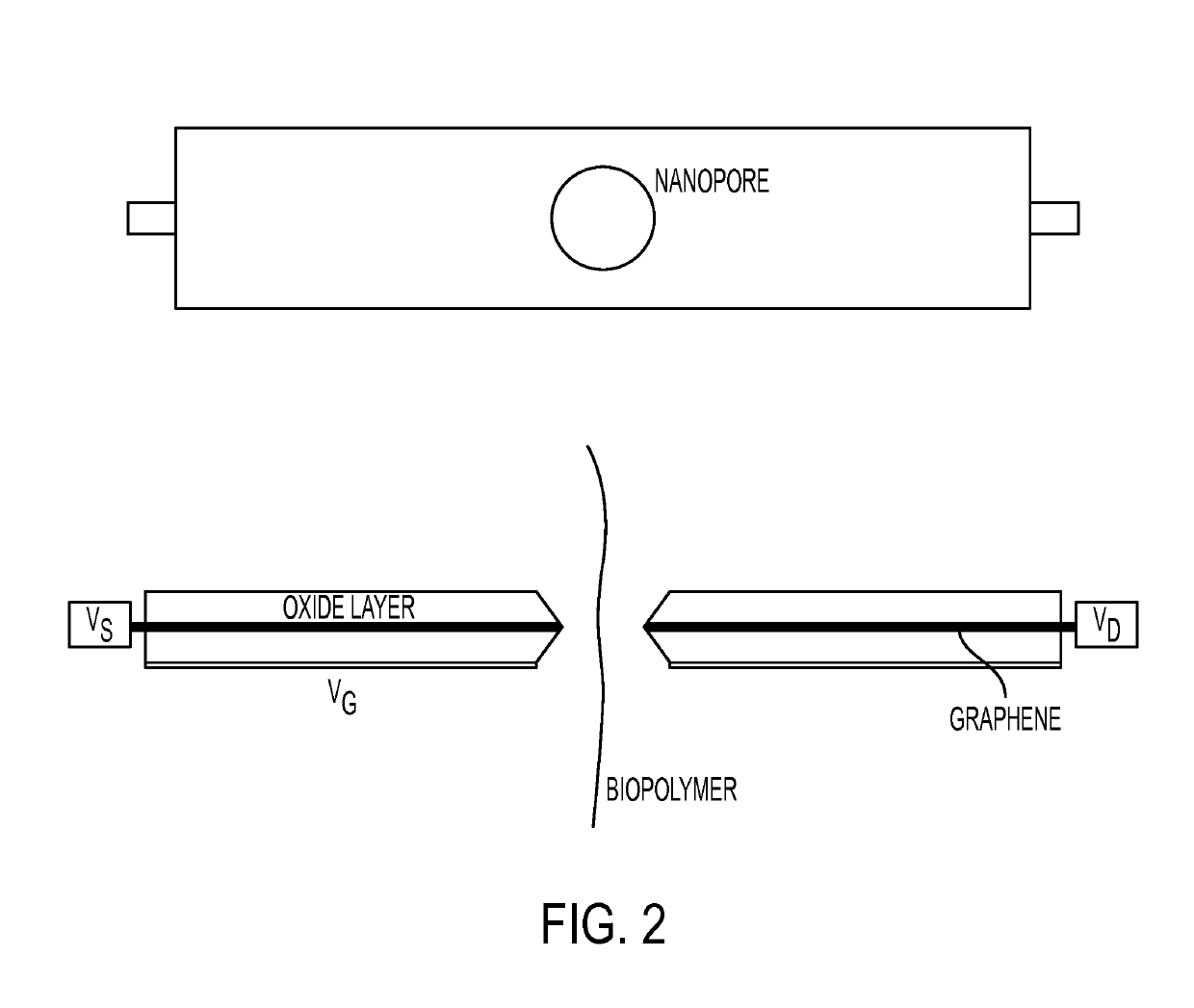
Nanopipette analysis of polymers
a polymer and nanopipette technology, applied in the field of nanopipette analysis of polymers, can solve the problems of challenging de novo assembly for large complex genomes, inability to sequence molecules from a single cell without amplification, and difficulty in deciphering the identity of molecules within complex mixtures, etc., to achieve low polymer rate, easy access to nanopipette, and minimize the lag time between measurements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Quartz Nanopipette Fabrication
[0127]In some embodiments, nanopipettes are fabricated from quartz capillaries with filaments, with an outer diameter of 1.0 mm and an inner diameter of 0.70 mm (QF100-70-5; Suffer Instrument Co.). In some embodiments, the capillary are pulled using a P-2000 laser puller (Suffer Instrument Co.) preprogrammed to fabricate nanopipettes with an inner diameter of approximately 50 nm. Parameters used are: Heat 625, Filament 4, Velocity 60, Delay 170, and Pull 180. In some embodiments, a solution of 10 mM buffer and 100 mM KCl, the pipettes give a current between −2500 and −4000 pA at a potential of −0.5 V.
Measurement Setup
[0128]In some embodiments, for measuring ionic current through a nanopipette, a two electrode setup is used. In some embodiments, a nanopipette is backfilled with buffer solution and an Ag / AgCl electrode inserted. In some embodiments, another Ag / AgCl electrode is placed in 0.3 mL bulk solution acting as auxiliary / reference electrode. In som...
example 2
nalysis
[0167]It should be noted that information provided in one application below may be relevant to other applications below.
Mapping DNA Sequence
[0168]DNA is extracted so substantially long molecules can be retained, e.g., 1 kb and longer, preferably several 100 Kb in length. The DNA is arrayed on a surface so that one end can bind to the surface and the other end is free in solution. This can be achieved with a number of surface chemistries, particularly when the immobilized DNA is fragments of genomic DNA, which typically have exposed single stranded bases at the end or a free 3′ or 5′ OH or phosphate. In some instances the DNA is immobilised onto Poly-lysine, APTES, cyano vinyl silane coated glass surface (e.g., cover glass). In some instances the DNA is immobilised onto streptavidin coated cover glass. A variety of modified surfaces available from Microsurfaces Inc. are used from one experimental implementation to the next. High salt concentration, such as those used in the el...
PUM
| Property | Measurement | Unit |
|---|---|---|
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com