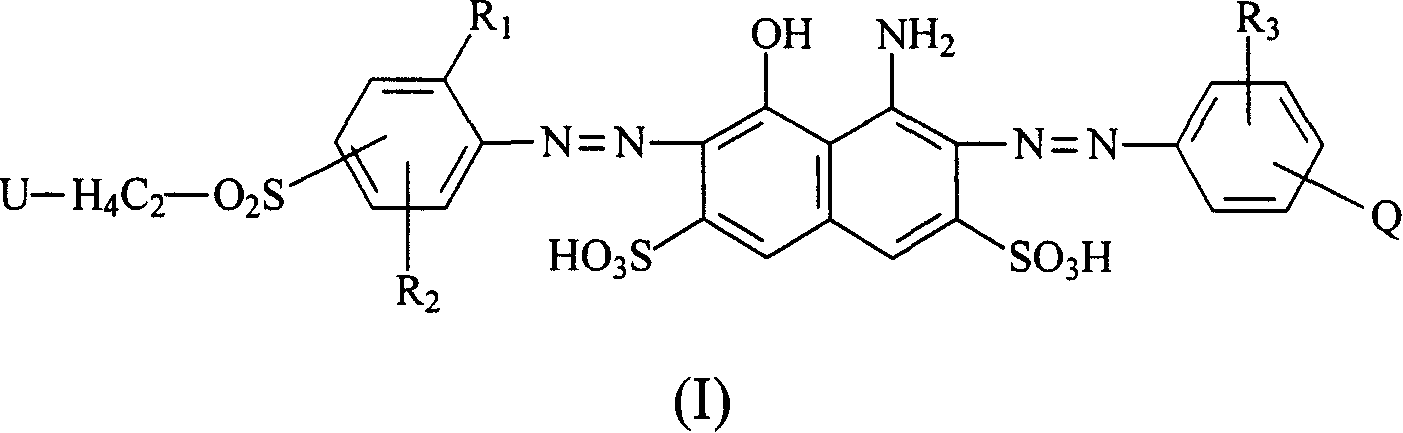
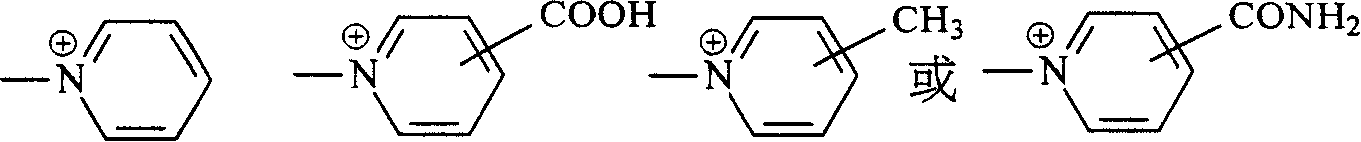Navy blue quaternary ammonium salt bisazo chemically-reactive dyes
A technology of reactive dyes and dye compounds, applied in the direction of azo dyes, reactive dyes, organic dyes, etc., can solve the problems of chlorine bleaching resistance and oxidation bleaching resistance to be improved, can not meet the market demand, etc., to achieve excellent chlorine resistance Effects of bleaching and oxidative bleaching resistance to washing fastness, excellent dyeing depth, and high fiber/dye stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0061] Take 31.1 parts of (2-methoxyl-4-β-sulfatoethylsulfone) aniline and disperse in 150 parts of water at 0°C, add 5 parts of NaOH, adjust the pH of the above solution to 13, keep the pH value stably for 15 minutes, then use HCl aqueous solution to adjust the reaction The pH of the solution is 5-6, and 12.7 parts of nicotine acid are added to the reaction solution. Then, the temperature of the reaction solution was raised to 60° C. and kept at the temperature for 1 to 2 hours, that is, the reaction was stopped. Finally, through the commonly used NaCl salting-out, filtration and brine washing procedures, a product with the structure of the following formula (R1) is obtained.
[0062]
preparation example 2
[0064] Take 36.1 parts of 1-aminobenzene-4-(β-sulfatoethylsulfone)-2-sulfonicacid and disperse in 150 parts of water at 0°C, and add 4.5 parts of NaOH to adjust the pH of the above solution to 13, keep the pH value for 15 minutes and then use HCl The aqueous solution adjusts the pH of the reaction liquid to 5-6, and then adds 12.7 parts of nicotine acid into the reaction liquid. Then, the temperature of the reaction solution was raised to 60° C. and kept at the temperature for 1 to 2 hours, that is, the reaction was stopped. Finally, a product with the structure of the following formula (R2) is obtained through the commonly used NaCl salting-out, filtration and brine washing procedures.
[0065]
preparation example 3
[0067] Take 32.5 parts of (2-methoxyl-4-β-sulfatoethylsulfone-5-methyl)aniline and disperse in 150 parts of water at 0°C, and add 4.5 parts of NaOH, adjust the pH of the above solution to 13, keep the pH stable for 15 minutes before using HCl The aqueous solution adjusts the pH of the reaction liquid to 5-6, and then adds 12.7 parts of nicotine acid into the reaction liquid. Then, the temperature of the reaction solution was raised to 60° C. and kept at the temperature for 1 to 2 hours, and a product with the structure of the following formula (R3) was obtained through the usual procedures of NaCl salting out, filtration and brine washing.
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com