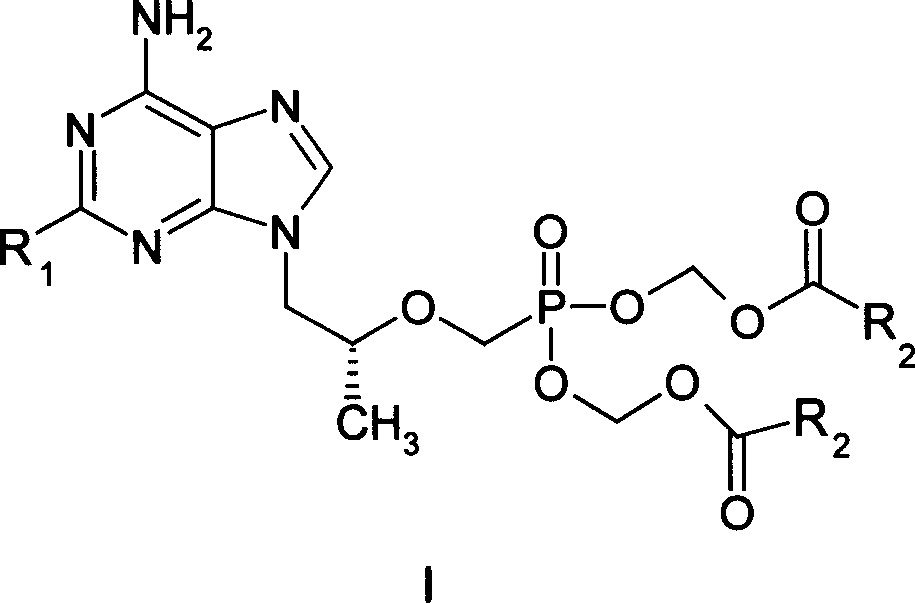
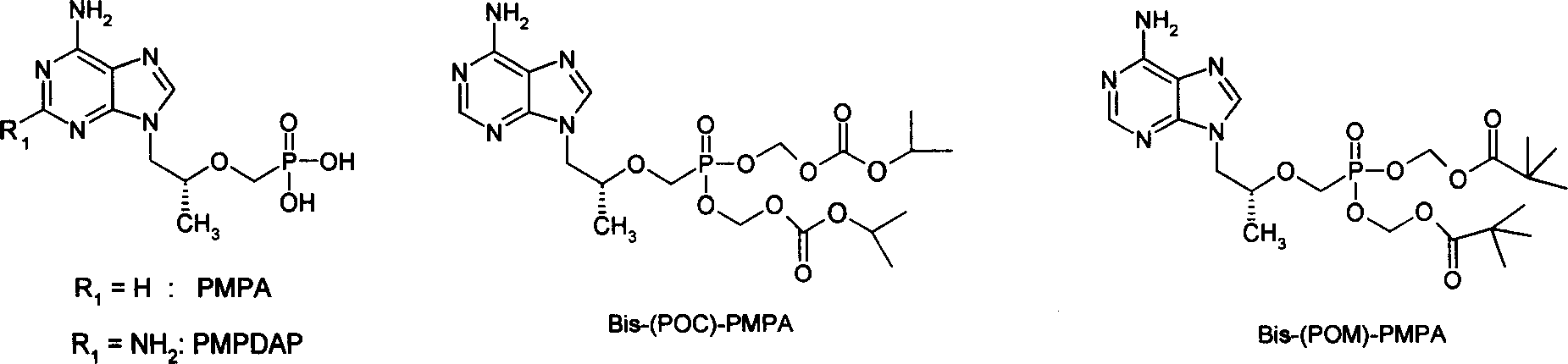
Precursor medicine of acyclic nucleoside phosphonic acid
A technology of prodrugs and drugs, which is applied in the fields of compounds of group 5/15 elements of the periodic table, pharmaceutical formulations, medical preparations containing active ingredients, etc. Constructs, low levels of carnitine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 (R)-9-{2-[bis-(isobutyryloxymethoxy)-phosphonomethoxy]-propyl}-adenine (I 1 ) preparation
[0018] 1.1 Synthesis of (R)-1-chloro-2-propanol
[0019] Add 208 g of (R)-lactate methyl ester to 1200 ml of dimethylformamide, cool in an ice bath, add 96 g of 60% sodium hydride in batches under stirring, and react with stirring for 1 hour. Then 408 g of benzyl bromide was added dropwise, and after the drop was completed, the mixture was stirred under ice bath for 4 hours, and continued to stir at room temperature for 48 hours. DMF was evaporated under reduced pressure, and the residue was separated by silica gel column chromatography, eluting with ethyl acetate:petroleum ether (1:9), and the desired components were collected and evaporated to dryness under reduced pressure to obtain 191 g of an oily liquid.
[0020] Dissolve 186 grams of the product from the previous step in 1200 ml of anhydrous tetrahydrofuran, place in an ice bath, add 60 grams of lithium alum...
Embodiment 2
[0035] Example 2 (R)-9-{2-[bis-(isovaleryloxymethoxy)-phosphonomethoxy]-propyl}-adenine (I 2 ) preparation
[0036] Referring to the method of Example 1.6, chloromethyl isovalerate was prepared by reacting isovaleryl chloride instead of isobutyryl chloride with thionyl chloride and paraformaldehyde.
[0037] With reference to the method of Example 1.7, (R)-PMPA reacts with chloromethyl isovalerate to obtain target compound I 2 . Elemental Analysis C 21 h 34 N 5 o 8 Calculated P value (%): C48.93, H6.65, N13.59; measured value (%): C49.03, H6.42, N13.41.
Embodiment 3
[0038] Example 3 (R)-9-{2-[bis-(2-methylbutyryloxymethoxy)-phosphonomethoxy]-propyl}-adenine (I 3 ) preparation
[0039] Referring to the method of Example 1.6, chloromethyl 2-methylbutyrate was prepared by reacting 2-methylbutyryl chloride instead of isobutyryl chloride with thionyl chloride and paraformaldehyde.
[0040] Referring to the method of Example 1.7, (R)-PMPA reacted with 2-methyl chloromethyl butyrate to obtain target compound I 3 . Elemental Analysis C 21 h 34 N 5 o 8 Calculated P value (%): C48.93, H6.65, N13.59; measured value (%): C48.79, H6.20, N13.85.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com