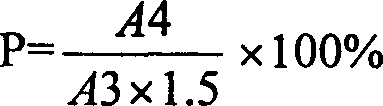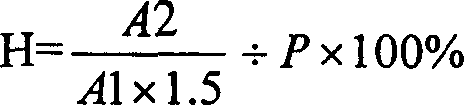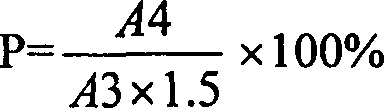Method for determining dissociation amylase content of pneumonia streptococcus capsular polysaccharide combo
A technique for Streptococcus pneumoniae and capsular polysaccharide, which is applied in the biological field and can solve the problems of inability to accurately measure the content of free polysaccharide of Streptococcus pneumoniae capsular polysaccharide conjugates and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Take the Streptococcus pneumoniae type 1 capsular polysaccharide conjugate as sample 1; take the derivative solution corresponding to the Streptococcus pneumoniae type 1 capsular polysaccharide conjugate to be tested as sample 3.
[0021] Another 4.5ml of Streptococcus pneumoniae type 1 capsular polysaccharide conjugate was taken and placed in a 50ml centrifuge tube; 4.5ml of the derivative solution corresponding to the Streptococcus pneumoniae type 1 capsular polysaccharide conjugate was taken and placed in another 50ml centrifuge tube. Add 1.125ml of 5mol / L sodium chloride solution to each centrifuge tube, shake and mix, then add 22.5ml of absolute ethanol, shake and mix, place at -20°C for 70 hours; centrifuge at 5°C, 4500rpm for 60 minutes, discard the supernatant liquid.
[0022] Add 0.75ml of water for injection (that is, containing 0% ethanol) to each centrifuge tube, shake and mix, and let stand at room temperature for 1 hour; then add 2.25ml of water for inject...
Embodiment 2
[0030] Take the Streptococcus pneumoniae type 2 capsular polysaccharide conjugate as sample 1, and take the derivative solution corresponding to the Streptococcus pneumoniae type 2 capsular polysaccharide conjugate to be tested as sample 3.
[0031] Another 4.5ml of Streptococcus pneumoniae type 2 capsular polysaccharide conjugate was taken and placed in a 50ml centrifuge tube; 4.5ml of derivative solution corresponding to the Streptococcus pneumoniae type 2 capsular polysaccharide conjugate was taken and placed in another 50ml centrifuge tube. Add 1.125ml of 5mol / L sodium chloride solution to each centrifuge tube, shake and mix, then add 22.5ml of absolute ethanol, shake and mix, place at -20°C for 90 hours; centrifuge at 5°C, 6500rpm for 90 minutes, discard the supernatant liquid.
[0032] Add 0.75ml of 60% ethanol solution to each centrifuge tube, shake and mix, and let stand at room temperature for 1 hour; then add 2.25ml of 60% ethanol solution, shake and mix, let stand a...
Embodiment 3
[0040] Take the Streptococcus pneumoniae type 3 capsular polysaccharide conjugate as sample 1, and take the derivative solution corresponding to the Streptococcus pneumoniae type 3 capsular polysaccharide conjugate to be tested as sample 3.
[0041] Another 4.5ml of Streptococcus pneumoniae type 3 capsular polysaccharide conjugate was taken and placed in a 50ml centrifuge tube; 4.5ml of derivative solution corresponding to the Streptococcus pneumoniae type 3 capsular polysaccharide conjugate was taken and placed in another 50ml centrifuge tube. Add 1.125ml of 5mol / L sodium chloride solution to each centrifuge tube, shake and mix, then add 22.5ml of absolute ethanol, shake and mix, place at -20°C for 90 hours; centrifuge at 5°C, 5500rpm for 70 minutes, discard the supernatant liquid.
[0042] Add 0.75ml of 40% ethanol solution to each centrifuge tube, shake and mix, and let stand at room temperature for 1 hour; then add 2.25ml of 40% ethanol solution, shake and mix, let stand a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com