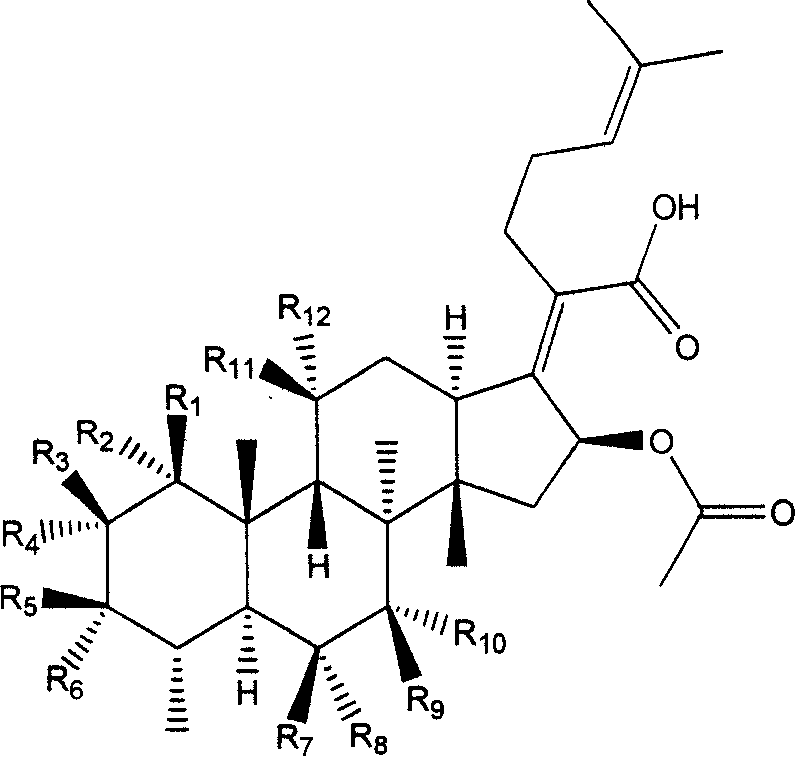
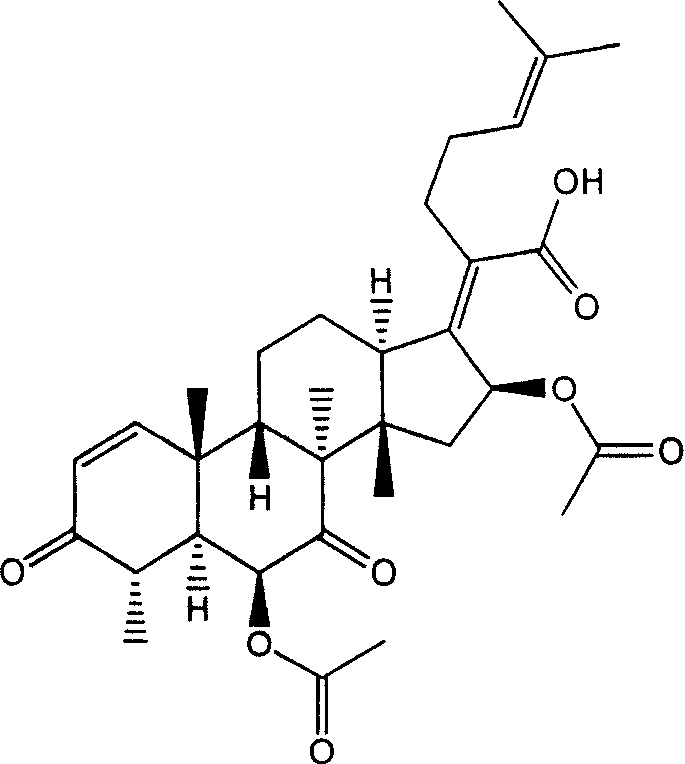
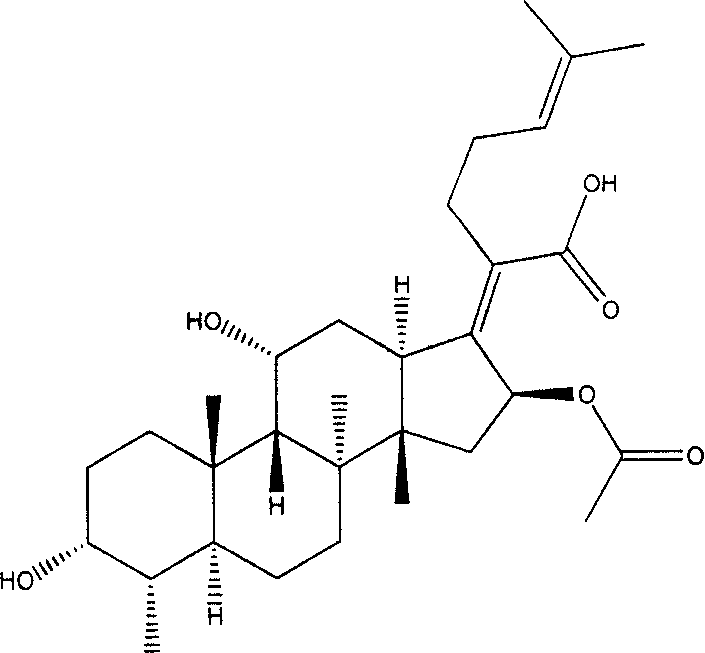
New use of fumigacin type steroids in producing medicine for anti-drug fast bacteria
A technology of fumagillic acid and drug-resistant bacteria, applied in the field of medicine, can solve problems such as the combination and use of fusidic acid and various antibiotics not involved, achieve obvious synergistic antibacterial effect, reduce side effects and adverse reactions, and achieve significant synergy synergistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 The in vitro antibacterial activity assay and antibacterial activity comparison of fumagic acid / chloramphenicol composition to clinical drug-resistant Staphylococcus aureus
[0036] 1 Materials and methods
[0037] 1.1 Clinical drug-resistant strains:
[0038] The clinical drug-resistant strains were multidrug-resistant Staphylococcus aureus (Staphylococcus.aureus) isolated from clinical patients by the Laboratory Department of the West China First Affiliated Hospital of Sichuan University, and the bacterial numbers were 13929, 12334, 11931, 12352, 12798, and 12760. These isolated strains were subcultured and used in this example.
[0039] 1.2 Drugs and reagents:
[0040] Erythromycin, streptomycin, chloramphenicol, kanamycin, gentamicin, and tetracycline are products of Amresco Company, biochemically pure; penicillin sodium is penicillin sodium for injection from Harbin Pharmaceutical Group General Pharmaceutical Factory, and the batch number is C 04071091...
Embodiment 2
[0051] Example 2 The in vitro antibacterial activity assay and antibacterial activity comparison of fumagic acid / erythromycin composition to clinical drug-resistant Staphylococcus aureus
[0052] According to the experimental method of Example 1, the antibacterial activity of erythromycin and fumonic acid / erythromycin composition against clinical drug-resistant Staphylococcus aureus was determined, and the results are shown in Table 2.
[0053] antibiotic name
Erythromycin MIC(μg / ml)
Fumonic acid (μg / ml)
0
1
2
4
try
test
name
say
Staphylococcus aureus 12334
Staphylococcus aureus 11931
Staphylococcus aureus 12352
Staphylococcus aureus 12798
Staphylococcus aureus 12760
1000
500
2000
2000
2000
16
8
64
125
500
0.25
0.125
1
2
8
0.0625
0.0625
0.0625
0.0625
0.0625
[0054] The above exp...
Embodiment 3
[0056] Example 3 The in vitro antibacterial activity assay and antibacterial activity comparison of the fumagillin / streptomycin composition to clinical drug-resistant Staphylococcus aureus
[0057] The antibacterial activity of streptomycin and fumonic acid / streptomycin composition against clinical drug-resistant Staphylococcus aureus was determined according to the experimental method of Example 1, and the results are shown in Table 3.
[0058] antibiotic name
Streptomycin MIC(μg / ml)
Fumonic acid (μg / ml)
0
1
2
4
test
Bacteria name
say
Staphylococcus aureus 12352
Staphylococcus aureus 12798
Staphylococcus aureus 12760
4
8
128
2
4
64
0.125
0.25
0.5
0.0625
0.0625
0.0625
[0059] The above experimental results show that the fumagic acid / streptomycin composition has a stronger antibacterial activity against clinical drug-resistant Staphylococcus...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com