Silinane compounds as cysteine protease inhibitors
A compound, heterocycloalkyl technology, applied in the field of cysteine protease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
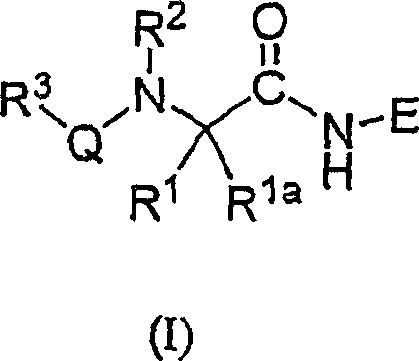
[0173] Although the broadest definition of the invention is given in the Summary of the Invention, certain compounds of the invention are preferred. For example:
[0174] A. A preferred group of compounds are those wherein E is -C(R 5 )(R 6 )X 1 compounds, of which:
[0175] R 5 is hydrogen or alkyl; and
[0176] R 6 is hydrogen, alkyl, -(alkylene)-OR 12 (where R 12 is hydrogen, alkyl or haloalkyl), cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heteroaryl, heteroaralkyl, heterocycloalkyl or heterocycloalkylalkyl, where aryl radical, aralkyl, heteroaryl, heteroaralkyl, heterocycloalkyl or heterocycloalkylalkyl is optionally selected from one, two or three independently selected from alkyl, haloalkyl, alkoxy, R of hydroxy, haloalkoxy, halogen, carboxy, alkoxycarbonyl, amino, monosubstituted amino, disubstituted amino or acyl a replace.
[0177] Preferred R 5 is hydrogen;
[0178] R 6 is an alkyl group, preferably ethyl or propyl, more preferably ethyl; and
[0179] ...
Embodiment 1
[0518] 1-(R)-morpholine-4-carboxylic acid [1-(4-cyano-1-ethylpiperidin-4-ylcarbamoyl)-2-(trimethylsilyl)ethyl]amide Synthesis
[0519]
[0520] step 1
[0521] (R)-2-Amino-3-trimethylsilylpropionic acid (0.320g, 2mmol) and N-methyl-N-trimethylsilyltrifluoroacetamide (MSTFA) (1.85g, 13mmol ) mixture was heated at 70 °C for 1 h. The reaction mixture was cooled and remaining MSTFA was removed in vacuo. Morpholinocarbonyl chloride (0.70ml, 6mmol) was added to the reaction mixture, heated at 70°C for 45 minutes, then cooled. Water and ice (25ml) were added to the reaction mixture and stirred until the evolution of carbon dioxide ceased. Extraction of this solution with ethyl acetate afforded 2-(R)-[(morpholine-4-carbonyl)amino]-3-(trimethylsilyl)propanoic acid (0.529 g), which was used without further purification in the following step.
[0522] step 2
[0523] To a solution of 2-(R)-[(morpholine-4-carbonyl)amino]-3-(trimethylsilyl)propanoic acid (140 mg, 0.51 mmol) in DM...
Embodiment 2
[0531] 1-(R)-morpholine-4-carboxylic acid [1-(4-cyano-1,1-dioxohexahydro-1λ6 Synthesis of -thiopyran-4-ylcarbamoyl)-2-(trimethylsilyl)ethyl]amide
[0532]
[0533] At room temperature, to the crude product 1-(R)-morpholine-4-carboxylic acid [1-(4-cyanothiopyran-4-ylcarbamoyl)-2-(trimethylsilyl) To a solution of ethyl]amide (260mg, 0.51mmol) in MeOH (15ml) was added potassium persulfate (469mg, 0.76mmol) in water (15ml). After 2 h, methanol was removed in vacuo and the residue was extracted with ethyl acetate. The ethyl acetate layer was washed with brine, dried and concentrated. The residue was purified by silica gel chromatography to give the title compound (47mg). h 1 NMR (DMSO-d 6 ): δ8.39(1H, s), 6.5(1H, d, J=7.6Hz), 4.1(1H, m), 3.49(4H, t, J=4.4Hz), 3.4-3.1(6H, m) , 2.7-2.55 (2H, m), 2.5-2.4 (4H, m), 1.05-0.85 (2H, m), 0.008 (9H, s). MS: 429.2 (M-1), 43 1.1 (M+1), 453.2 (M+Na).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com