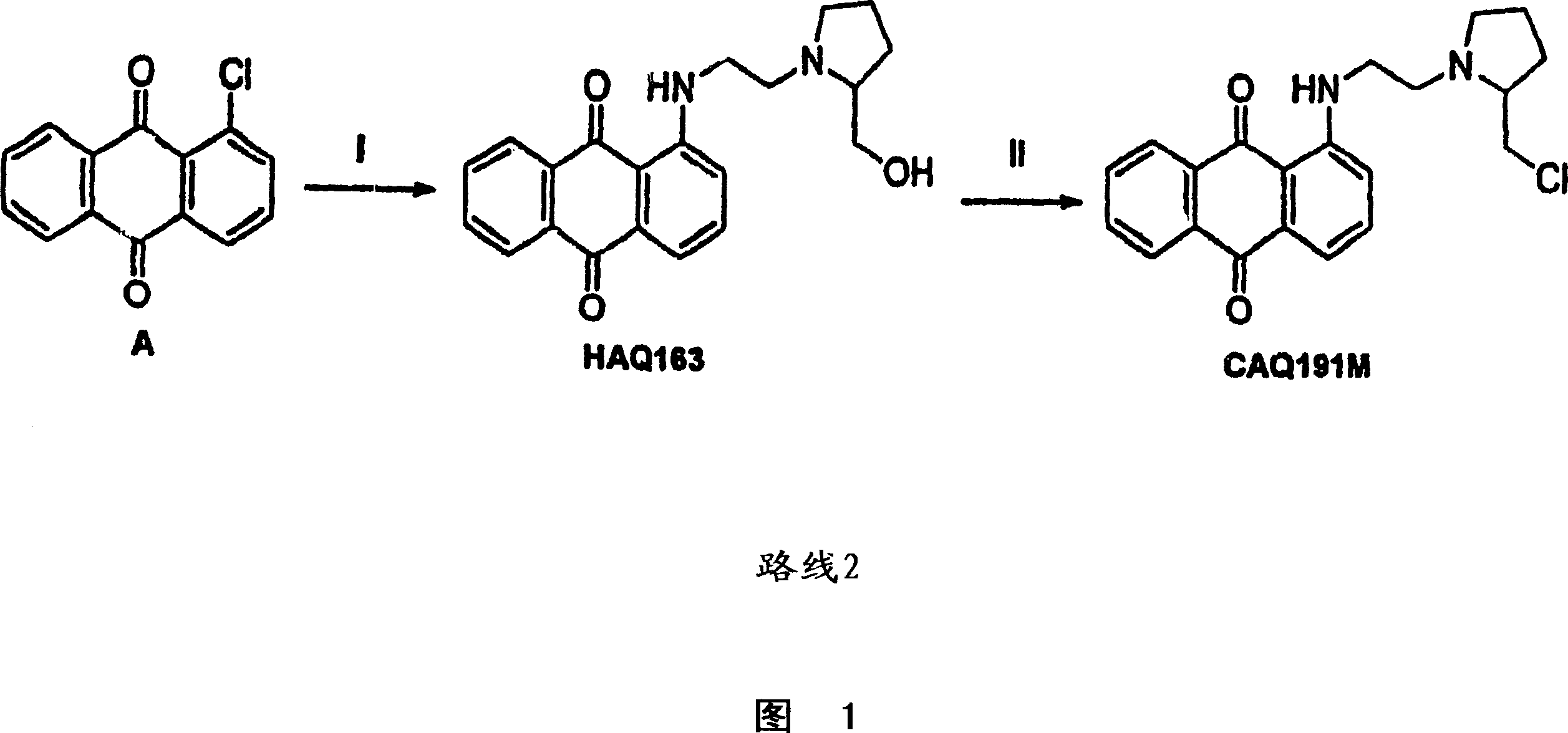
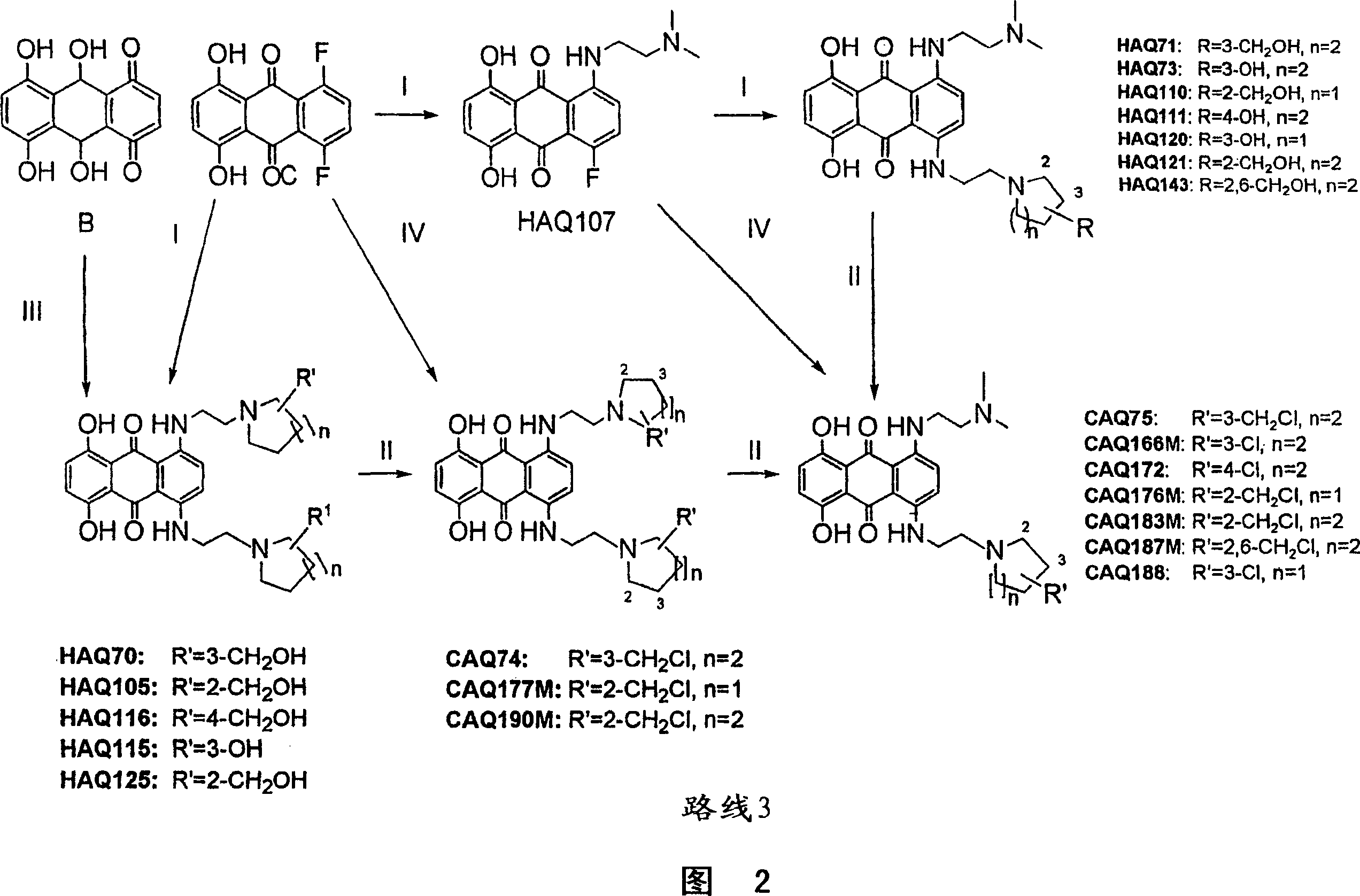
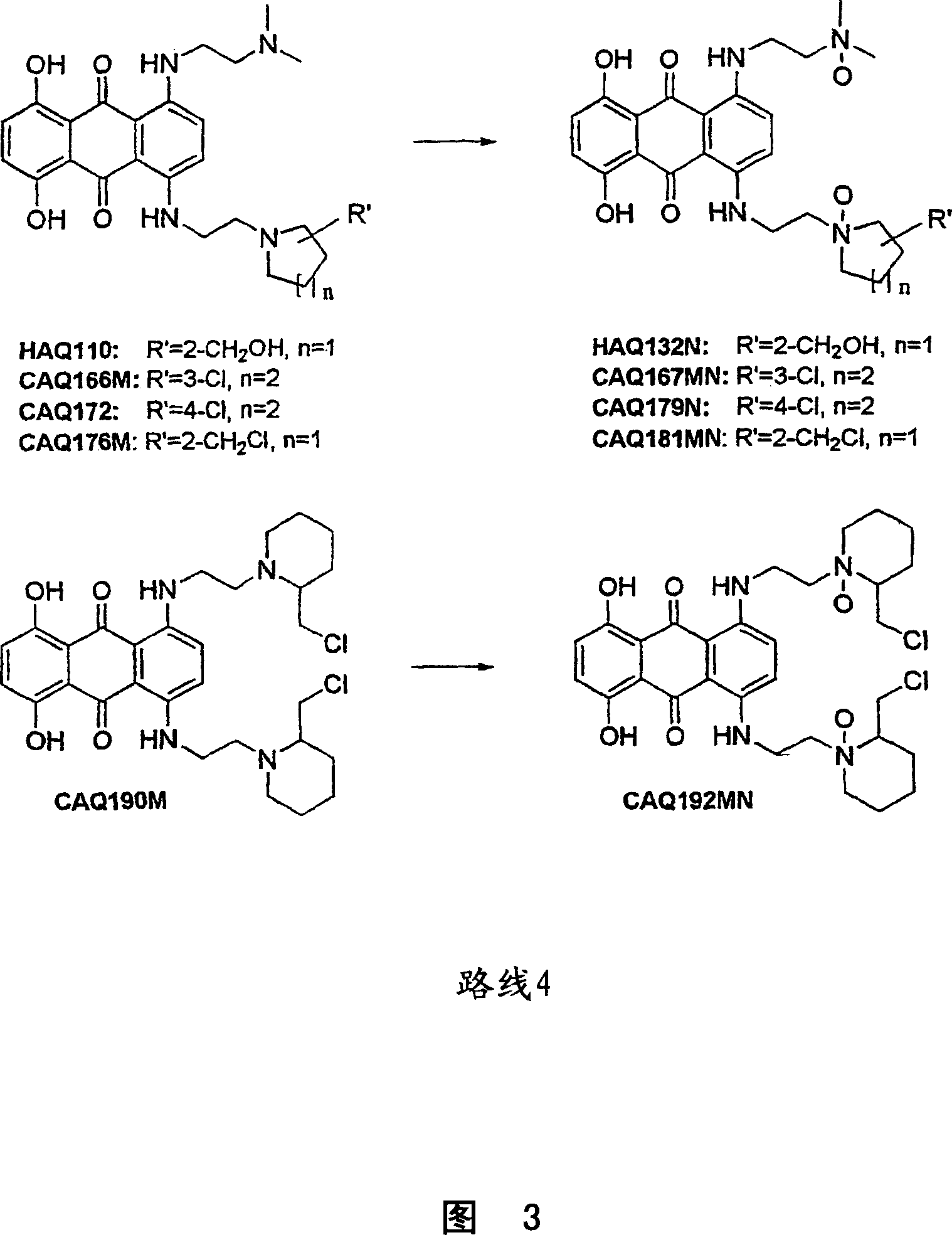
Anthraquinone compounds as anti cancer compounds
A compound, anthraquinone technology, applied in the field of N-oxides, can solve the problems of compound instability, inability to separate, difficult to separate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and 2
[0146] Amination of leucoquinizarin and 5,8-dihydroxyquinizarin
[0147] 1-{[(2-dimethylamino) ethyl] amino}-4-{[2-(3-hydroxypiperidin-1-yl) ethyl] amino}-anthracene-9,10-dione ( HAQ22) and 1,4-bis-{[2-(3-hydroxypiperidin-1-yl)ethyl]-amino}-anthracene-9,10-dione (HAQ24)
[0148] Pass N 2 Next, a solution of N-N-dimethylethylenediamine (0.92 g, 10.42 mmol) and 1-(2-aminoethyl)-3-piperidin-3-ol (1.50 g, 10.42 mmol) in EtOH (5 mL) Simultaneously, a suspension of leucoquinizarin (0.63 g, 2.61 mmol) in EtOH (25 mL) was added. After stirring for 8 h at reflux temperature, the reaction mixture was stirred for an additional 14 h at room temperature. EtOH was removed in vacuo and the residue was added to ice-cold brine. The precipitated solid was isolated by filtration and lyophilized. Pass the dark blue solid through a short column of flash chromatography: first use CH 2 Cl 2 Remove non-polar by-products, then gradually increase the polarity to remove more by-products, and fina...
Embodiment 3
[0153] 1,4-bis-{[2-(3-hydroxymethylpiperidin-1-yl)ethyl]amino}-5,8-dihydroxyanthracene-9,10-dione (HAQ70)
[0154] Pass N 2[1-(2-Aminoethyl)-piperidin-3-yl]methanol (1.9 g, 12 mmol) in EtOH (2 mL) was added to stirring 5,8-dihydroxyquinizarine leuco (272 mg, 1 mmol) in EtOH (15 mL). After stirring at reflux temperature for 7 h, the reaction mixture was cooled to room temperature and stirred for an additional 16 h. EtOH was removed in vacuo and the residue was added to ice-cold brine. The precipitated solid was isolated by filtration and lyophilized. use CH 2 Cl 2 Then gradually increase the polarity to CH 2 Cl 2 / CH 3 OH (4:1), the dark blue solid was subjected to flash chromatography. then CH 2 Cl 2 / CH 3 OH / NH 3 (94:6:0.75) was used as eluent to subject the crude product to flash chromatography. The title compound (115.9 mg, 21%) was obtained as a dark blue solid. Melting point 180-183°C; δ H (250MHz; CDCl 3 ); 1.1-1.95(m, 12H, 2xOH and 10x ring-H), 2.25(m, ...
Embodiment 4
[0156] 1,4-bis-{[2-(3-hydroxymethylpiperidin-1-yl)ethyl]amino}-anthracene-9,10-dione (HAQ38)
[0157] Although not shown in Scheme 3, leucoquinizarin (0.23g, 0.95mmol), [1-(2-aminoethyl)-piperidin-3-yl]methanol (0.06g, 3.8mmol) and Ethanol (25mL), the same method as HAQ70. The title compound (0.15 g, 29%) was obtained as a dark blue solid. Melting point 112-114°C; δ H (250MHz; CDCl 3 ); 1.15(m, 2H, 2xCH 2 CHCH 2 OH), 1.5-1.75(m, 6H, 2xCH 2 CH 2 CH and 2xCH 2 CH 2 CH 2 ), 1.85(m, 2H, 2xCH 2 CH 2 CH), 2.3(m, 4H, 4xring-H), 2.5(s(b), 4H, 2xring-H, 2xOH), 2.65(t, 4H, J=5Hz, 2xHNCH 2 CH 2 N), 2.75(2xd, 2H, J=3Hz and 12Hz, 2x ring-H), 3.42(q, 4H, J=5Hz, 2xHNCH 2 CH 2 N), 3.55 (2xd, 2H, J=5Hz and 12Hz, 2xCHCH 2 OH), 3.7 (2xd, 2H, J=9Hz and 12Hz, 2xCHCH 2 OH), 7.1(s, 2H, C(2)H and C(3)H), 7.6(m, 2H, C(6)H and C(7)H), 8.3(m, 2H, C(5) )H, C(8)H) and 10.75(t, 2H, C(1)NH and C(4)NH); δ C (62.9MHz; CDCl 3 ); 24.08, 26.92, 37.90, 40.52, 54.43, 56.95, 57.75, 65.70, 109.89...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com