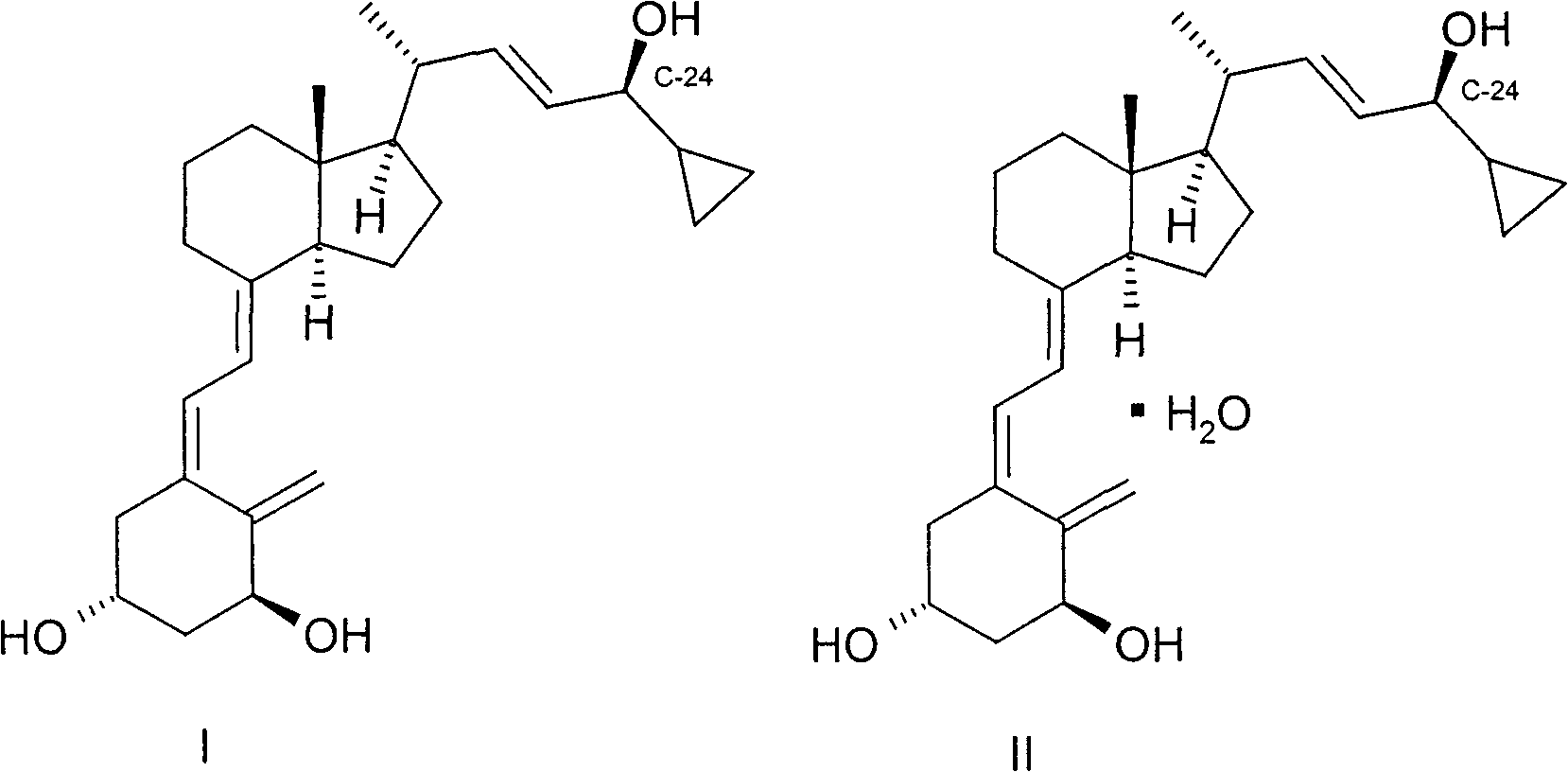
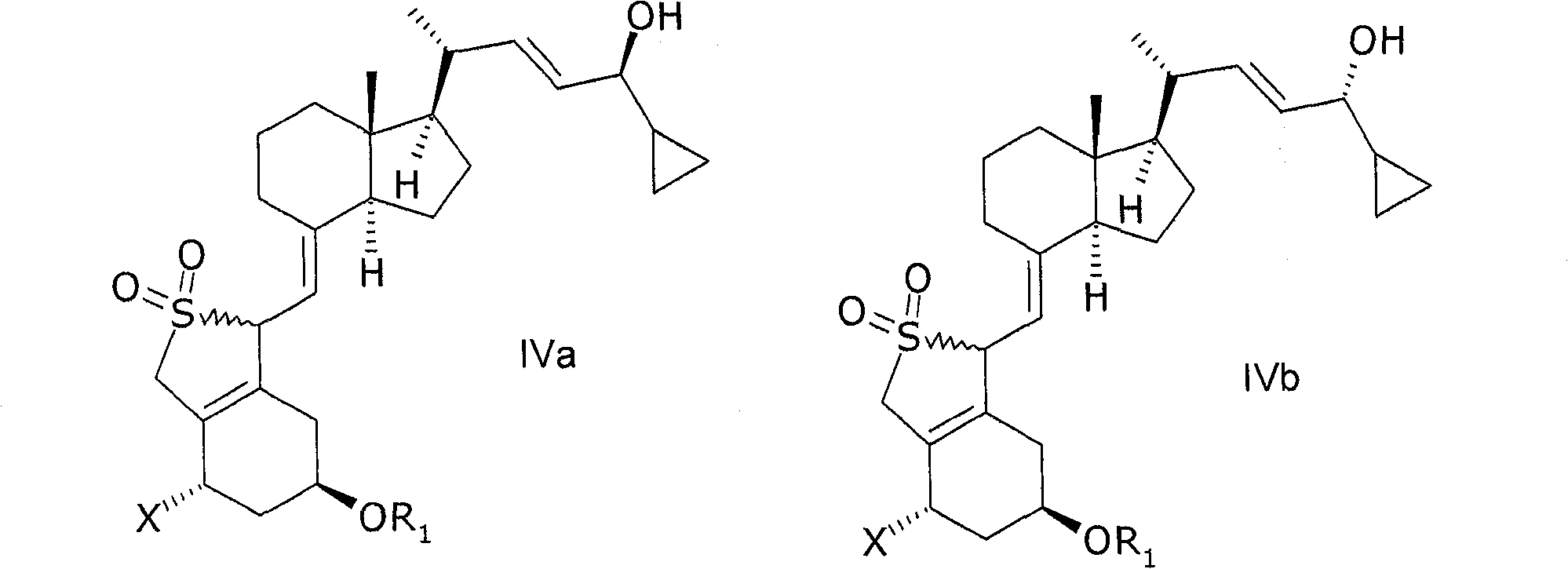
Stereoselective synthesis of vitamin D analogues
A technology for compounds and mixtures, applied in the field of intermediates for preparing calcipotriol or calcipotriol monohydrate, can solve the problems of decreased yield, easy to be oxidized, lengthy processing procedures, etc., and achieve the effect of improving yield and efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
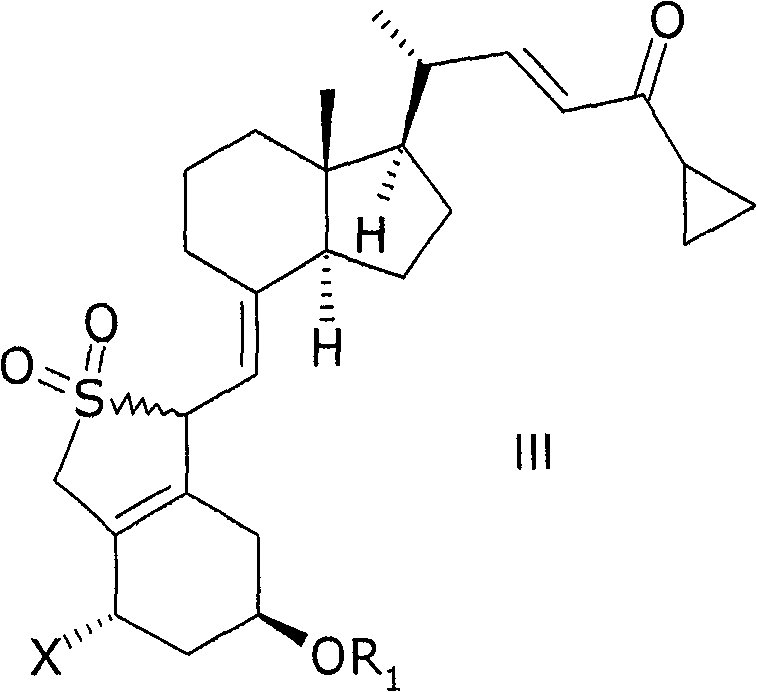
[0138] III: X=OR 2 , R 1 , R 2 = tert-butyldimethylsilyl
[0139] 1(S), 3(R)-bis(tert-butyldimethylsilyloxy)-20(R)-(3′-cyclopropyl-3′-oxoprop-1′(E)-ene base)-9,10-broken pregnant steroid (secopregna)-5(E), 7(E), 10(19)-triene SO 2 - adduct
[0140] 20(R), 1(S), 3(R)-bis(tert-butyldimethylsilyloxy)-20-(3'-cyclopropyl-3'-oxopropane-1'(E )-alkenyl)-9,10-depregna-5(E),7(E),10(19)-triene (according to M.J. Calverley, Tetrahedron, Vol. 43, No. 20, Nos. 4609-4619 Page, 1987 prepared by the method described in) (20.0g) was dissolved in toluene (210ml) at 20°C, then water (40ml) and SO 2 (20ml). After HPLC {from Merck's column LiChrosorb Si 60 5 μm, 250 * 4mm, the flow rate of 2ml / min, detection and mass detection at 270nm, hexane / ethyl acetate 9: 1 (volume ratio)} judged that the reaction had been completed (Usually after 2-2.5 hours), a mixture of sodium hydroxide (27.7%, 60ml) and water (80ml) was added at 10-18°C until the pH of the reaction mixture was 6. The toluene phas...
preparation example 1
[0142] VII: X=OR 2 , R 1 , R 2 = hydrogen
[0143] 1(S), 3(R)-dihydroxy-20(R)-(3′-cyclopropyl-3′-oxoprop-1′(E)-enyl)-9,10-depregna -5(E), 7(E), 10(19)-triene
[0144] 20(R), 1(S), 3(R)-bis(tert-butyldimethyl Silyloxy)-20-(3'-cyclopropyl-3'-oxoprop-1'(E)-enyl)-9,10-depregna-5(E),7(E) , 10(19)-triene was dissolved in acetonitrile. Aqueous hydrofluoric acid (40%) was added and the mixture was stirred at room temperature for about 1 hour. The progress of the reaction was conveniently checked by TLC using ethyl acetate as eluent. Ethyl acetate was added to the reaction mixture and the mixture was washed with aqueous sodium hydroxide. MgSO for organic phase 4 Dried and concentrated. The formed crystals (white needles) were filtered off, washed with ethyl acetate and dried in vacuo to afford the title compound VII (X=OR 2 , R 1 , R 2 = hydrogen). 1 H NMR (CDCl 3 )VII / X=OR 2 , R 1 , R 2 = Hydrogen = 6.77(dd, 1H), 6.57(d, 1H), 6.15(d, 1H), 5.88(dd, 1H), 5.13(dd, 1H), ...
Embodiment 2
[0146] III: X=OR 2 , R 1 , R 2 = hydrogen
[0147] 1(S), 3(R)-dihydroxy-20(R)-(3′-cyclopropyl-3′-oxoprop-1′(E)-enyl)-9,10-depregna -5(E), 7(E), 10(19)-triene SO 2 - Adducts.
[0148] The method is the same as in Example 1, except that the starting material is 1(S), 3(R)-dihydroxy-20(R)-(3'-cyclopropyl-3'-oxo) prepared in Preparation Example 1 Prop-1'(E)-enyl)-9,10-segreg-5(E),7(E),10(19)-triene. 1 H NMR (CDCl 3 )III / X=OR 2 , R 1 , R 2 = Hydrogen δ = 6.80(dd, 1H), 6.15(d, 1H), 4.75(m, 2H), 4.5-3.9(m, 4H), 3.70(d, 1H), 2.60(m, 1H), 2.5- 0.8(m, 25H), 0.68(s, 3H)ppm; 13 C NMR (CDCl 3 )III / X=OR 2 , R 1 , R 2 = Hydrogen δ = 201.0, 152.1, 151.0, 133.7, 129.2, 128.3, 108.8, 67.3, 65.1, 63.6, 56.1, 55.9, 55.5, 46.5, 40.1, 39.9, 33.9, 29.8, 27.4, 23.9, 22.1, 19.5, 18.9, 12.2, 11.2ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com