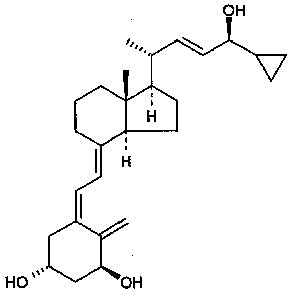
Calcipotriol nanosuspension
A technology of suspension and calcipotriol monohydrate, which is applied in the field of nanocrystal suspension, can solve problems such as aggravating inflammatory response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0050] The present suspension may contain non-ionic, polymeric surfactants, especially isopropyl oleate, which are added to better prevent aggregation of nanocrystals of calcipotriol and / or to prevent crystal growth. The surfactant should preferably be one that does not cause any significant solubilization of the calcipotriol monohydrate nanocrystals, i.e. it should have poor solubilizing power, and may advantageously be selected from poloxamer Or polysorbate surfactant, and polyoxyethylene C6-24 alkyl ether. The poloxamer may be selected from poloxamer 124, poloxamer 188, poloxamer 237, poloxamer 338 and poloxamer 407. In particular, it has been found that Poloxamer 188 has poor solubilizing power for solubilizing calcipotriol and thus serves as the currently favored surfactant for the present nanosuspensions. When polysorbate is used as the surfactant, it may be selected from polysorbate 20, polysorbate 40, polysorbate 60, polysorbate 61, polysorbate 80 and polysorbate 81. ...
Embodiment 1
[0141] Preparation of Calcipotriol Monohydrate Nanocrystals
[0142] 4 g of Poloxamer 188 and 4 g of isopropyl oleate were dissolved in 196 ml of laboratory water while stirring, and an appropriate amount of NaOH was added to adjust the pH to 8.5.
[0143] Weigh 3.5g of 2mm glass spheres into a vial fitted with a screw cap. 0.035 g of calcipotriol monohydrate was added to each vial, followed by 1.05 g of a solution of 2% poloxamer 188 and 2% isopropyl oleate was added to each vial. Calcipotriol monohydrate was ground by shaking on an orbital shaker at 2000 rpm.
[0144] After grinding, the vial and glass ball used for grinding were washed with 24.0 g of laboratory water pH 8.5, and the suspension of calcipotriol monohydrate was poured into a blue cap bottle. The suspension was transferred to an Emulsiflex C3 (Avestin) high pressure homogenizer and the blue cap bottle was washed with 4.9 g of laboratory water pH 8.5. High pressure homogenization was carried out at 500 bar fo...
Embodiment 2
[0146] Ointment Containing Calcipotriol Monohydrate Nanocrystals
[0147] An ointment of the composition shown in Table 1-1 below is prepared by mixing the components of the lipid phase (hydrocarbons + polyoxyethylene-2-stearyl ether + α-tocopherol) under heating to 80-85° C. + isopropyl oleate). Disodium edetate and disodium hydrogen phosphate dihydrate were dissolved in an appropriate amount of calcipotriol monohydrate nanosuspension adjusted to contain 50 μg / g calcipotriol monohydrate (as in Example 1) to prepare the aqueous phase. With stirring and heating to 35-40 °C, glycerol was added to the suspension and the pH of the mixture was adjusted to 8.5 with 1N HCl or NaOH as needed.
[0148] Add the aqueous phase to the lipid phase and stir for 30 minutes. After that, the obtained ointment was cooled slowly to below 32°C, packed into aluminum tubes and stored at room temperature.
[0149] Composition (mg / g)
[0150] Evaluation of the suspension
[0151]
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com