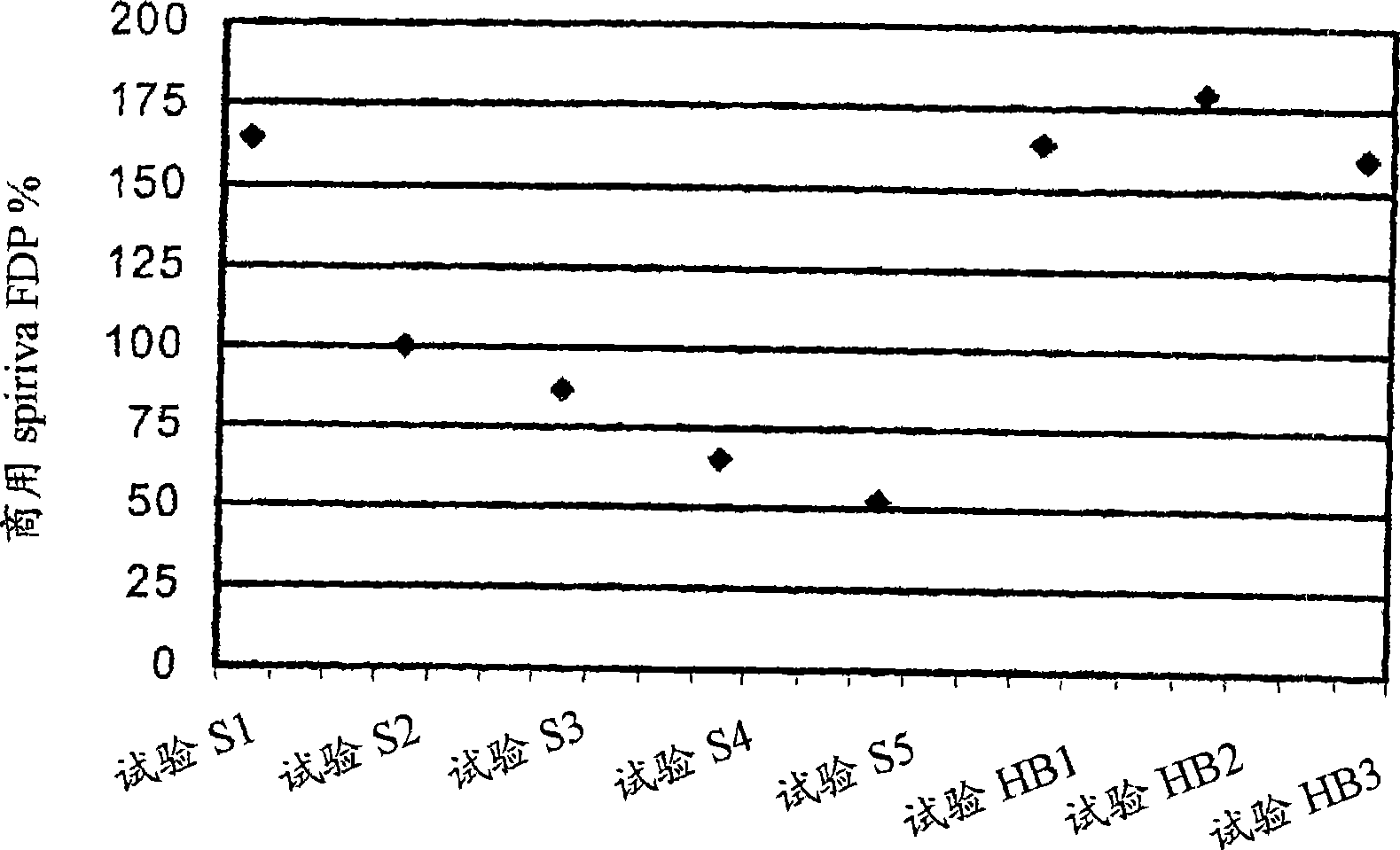
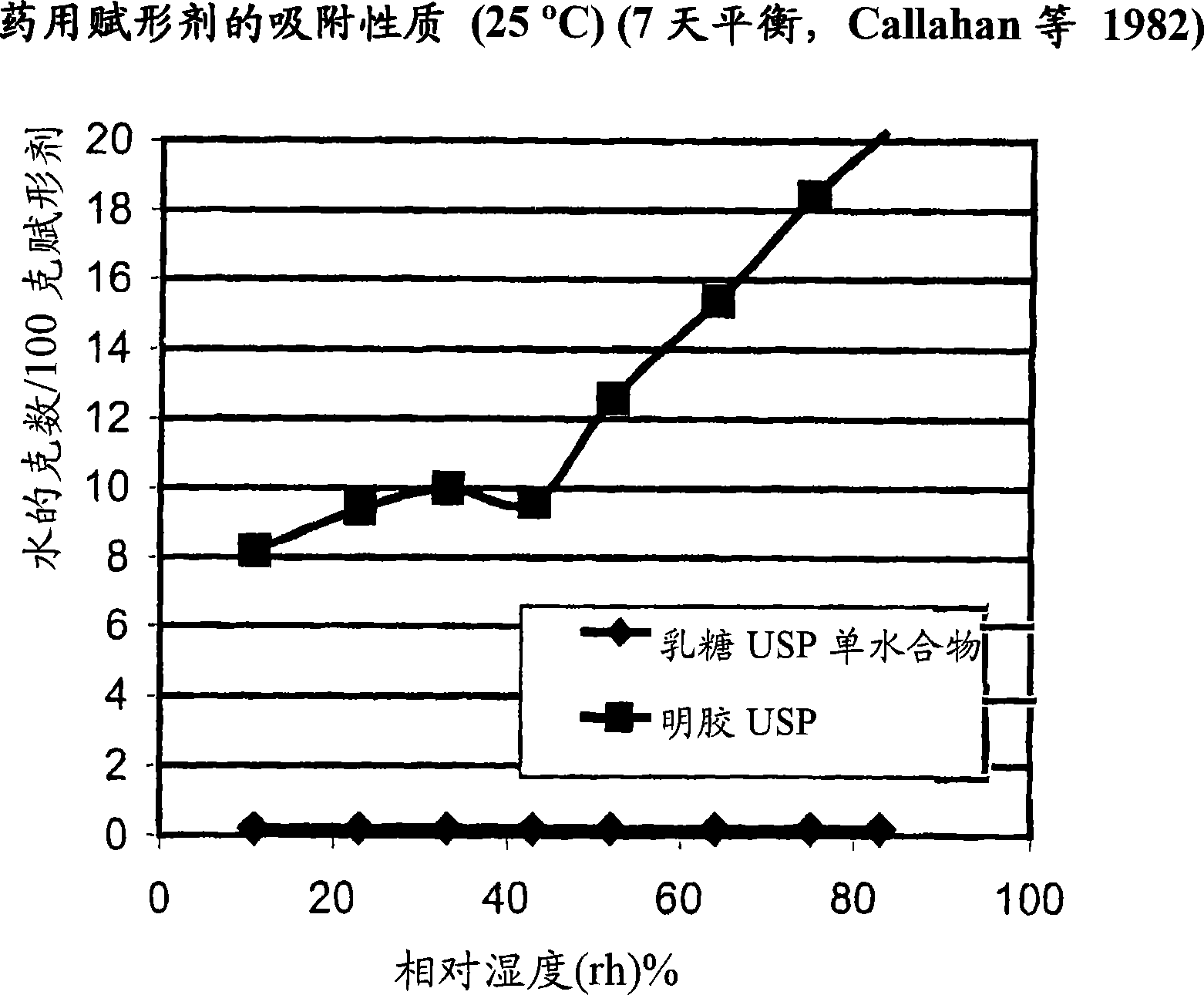
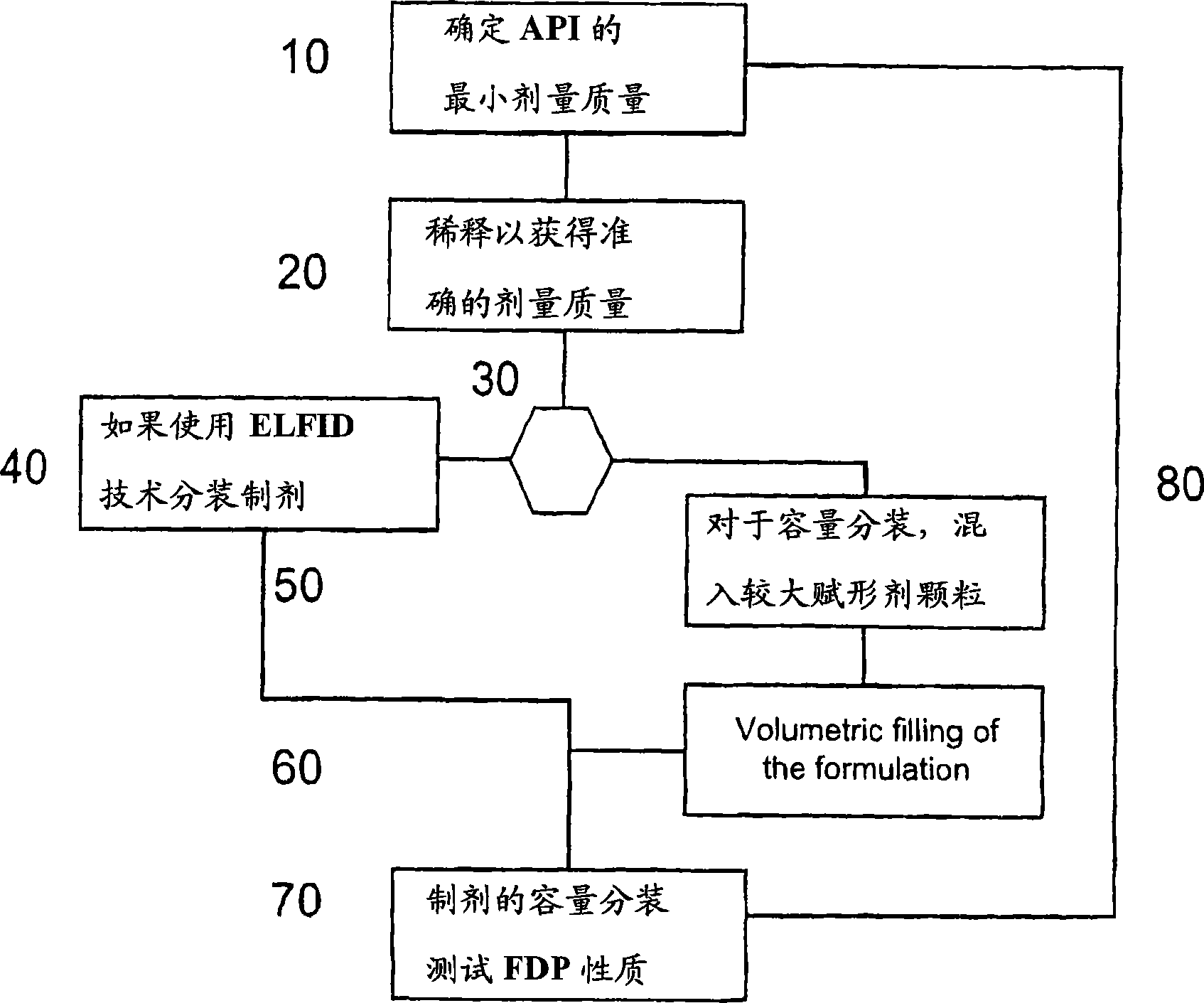
Pre-metered dry powder inhaler for moisture-sensitive medicaments
A dry powder inhaler, a predetermined amount of technology, applied in the field of dry powder inhaler
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0029] The present invention relates to DPIs loaded with moisture sensitive drugs, preferably comprising tiotropium, and describes dose release to achieve high levels of release FPD. Preferably, the DPI is a predetermined amount. In addition, the present invention addresses how this sensitive drug can be protected throughout all stages of storage, transport, dispensing, re-storage and final administration from the moment the dose is formed and sealed to the moment the user inhales the selected dose No moisture problem. Suitable dry powder inhalers for moisture sensitive drugs are also disclosed.
[0030] The present invention discloses a dry moisture-proof, direct loading and sealing container containing a dose of tiotropium in a high FPD formulation containing at least one excipient. The term "tiotropium" is a generic term for all its active forms, including pharmaceutically acceptable salts (especi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com