Prepn of carbapenum type antibiotic Faropenum sodium
A technology of faropenem sodium and compounds, which is applied in the field of preparation of carbapenem antibiotic faropenem sodium, and can solve the problems of complex preparation, low yield, harm to human body and environment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
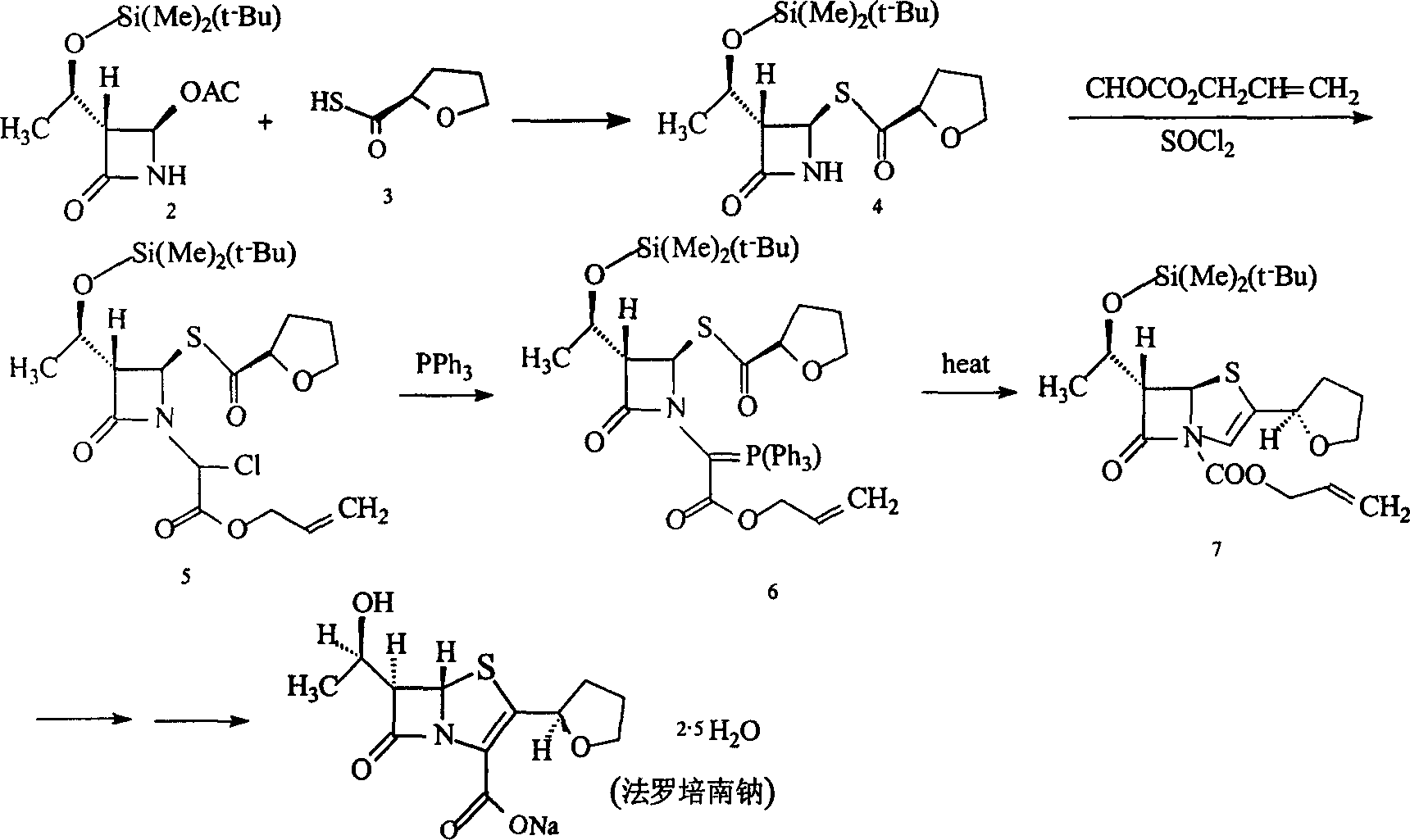
Image
Examples
Embodiment 1
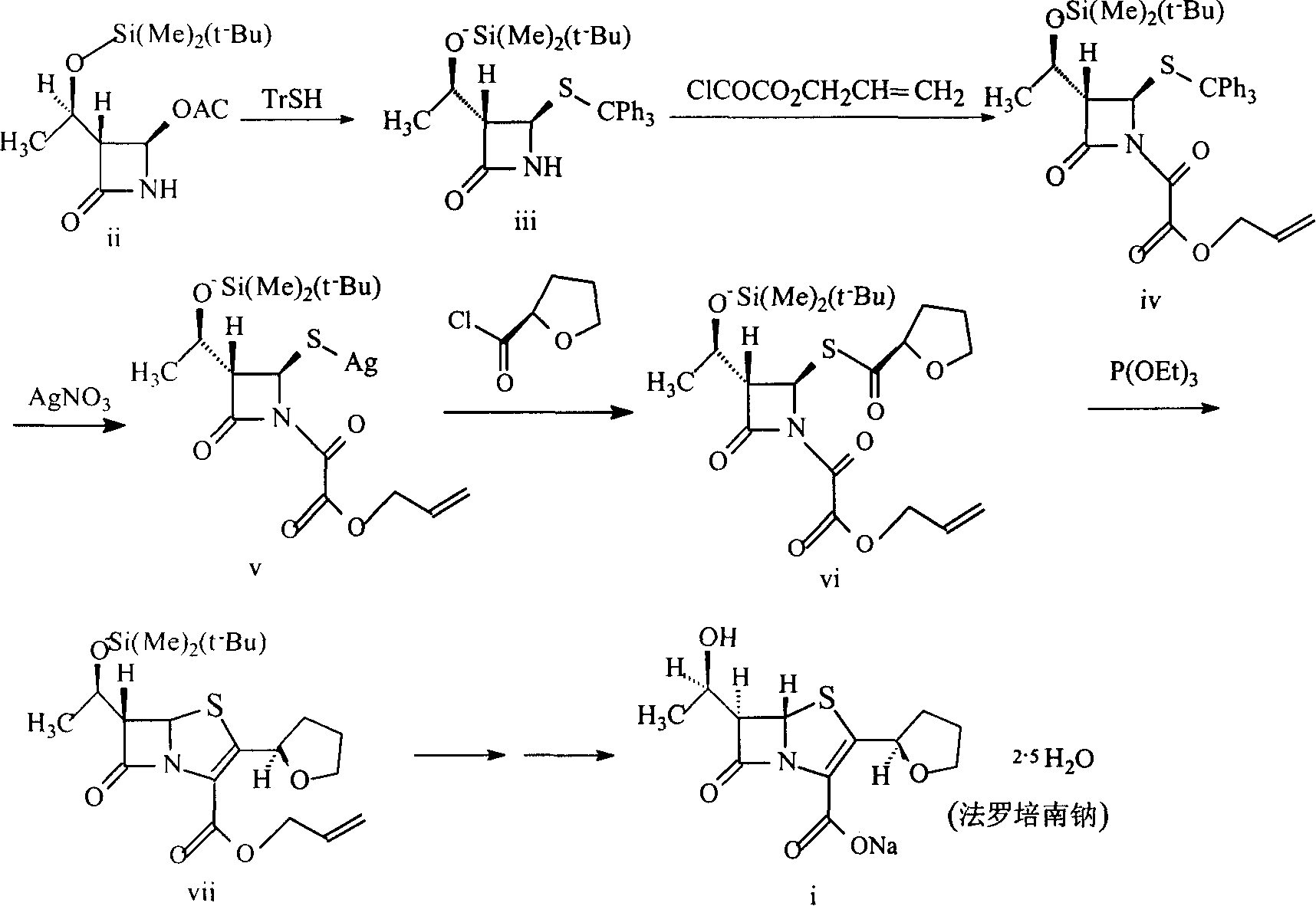
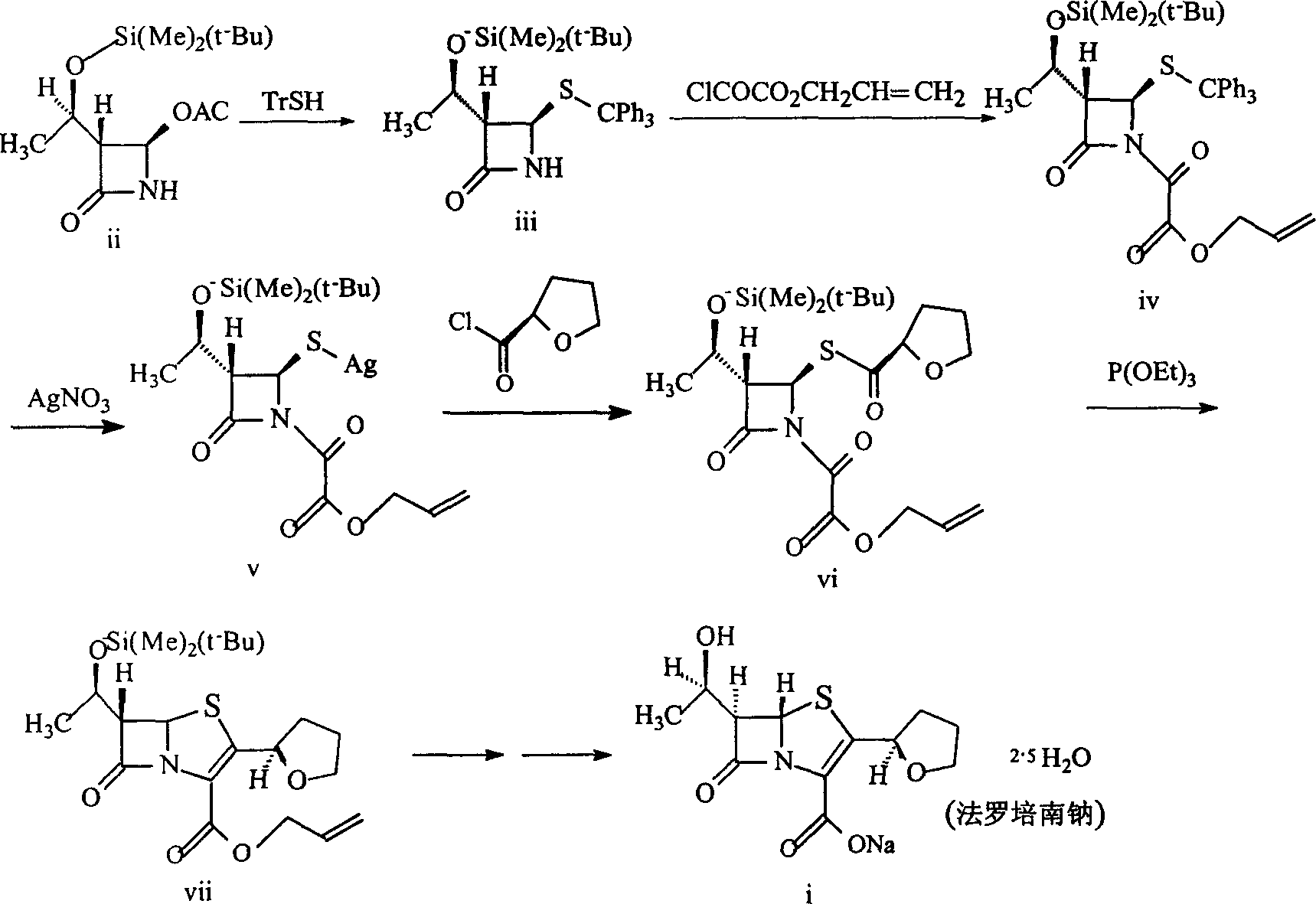
[0088] Example 1. Preparation of (3S, 4R)-3-[(R)-1-tert-butyldimethylsiloxyethyl]-4-tritylthio-azetidin-2-one (III )
[0089]
[0090] Dissolve 7.65g of sodium hydrogen in 164ml of DMF, add dropwise to 52.77g of triphenylmercaptan (294.35) / 287ml of DMF solution at 0°C, add dropwise within 0.5 hours, stir the mixture for 10 minutes, then add within 15 minutes 50g 4AA / 164ml DMF solution, kept at 0°C, continued to stir for 45 minutes, poured the reaction mixture into 900g of saturated ammonium chloride ice solution, extracted with 1000ml of ether (three times), washed the ether layer twice with water, and washed with saturated brine Twice, dried over anhydrous sodium sulfate, evaporated to dryness, and the solid residue was washed twice with 300ml ether, sucked dry, dried, and weighed to obtain 72g of solid, with a yield of 82.2%. Melting point: 94-96°C
[0091] TLC: petroleum ether: ethyl acetate = 4: 1 Rf = 0.3
Embodiment 2
[0092] Example 2. Preparation of (3S, 4R)-1-(allyloxyoxalyl)-3-[(R)-1-tert-butyldimethylsiloxyethyl]-4tritylthio-nitrogen Heterobutan-2-one (IV)
[0093]
[0094]
[0095] In a 1000ml three-neck flask, add 50g "III" and 50ml dichloromethane to dissolve, cool to -10°C in an ice-salt bath, add dropwise a solution of 25g allyl oxalyl chloride in dichloromethane (40ml), and then drop Add a solution of 18g of triethylamine in dichloromethane (40ml), keep the dropwise addition temperature not exceeding -5°C, react the mixture at -5°C--10°C for 1.5 hours, add water (150ml) to dilute, and dichloromethane (100ml) Extracted, combined organic phases, washed with water, washed with saturated sodium bicarbonate solution, dried over anhydrous sodium sulfate, and rotary evaporated to obtain 58 g of a light yellow solid with a yield of 95%.
[0096] TLC: petroleum ether: ethyl acetate = 4: 1 Rf = 0.4
Embodiment 3
[0097] Example 3. Preparation of (3S, 4R)-1-(allyloxyoxalyl)-3-[(R)-1-tert-butyldimethylsiloxyethyl]-4-thiosilver-aza Cyclobutan-2-one (V)
[0098]
[0099] The above product "IV" was dissolved in 1000ml of methanol, and 2ml (25mmol) of pyridine and a solution of silver nitrate (100g) in methanol (500ml) were added. The mixture was stirred at below 10°C in the dark for 3 hours, concentrated and extracted with dichloromethane (1000ml). The organic layer was washed with water (800ml), dried over anhydrous magnesium sulfate, and evaporated to dryness to obtain the silver salt. It was directly used in the next reaction without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com