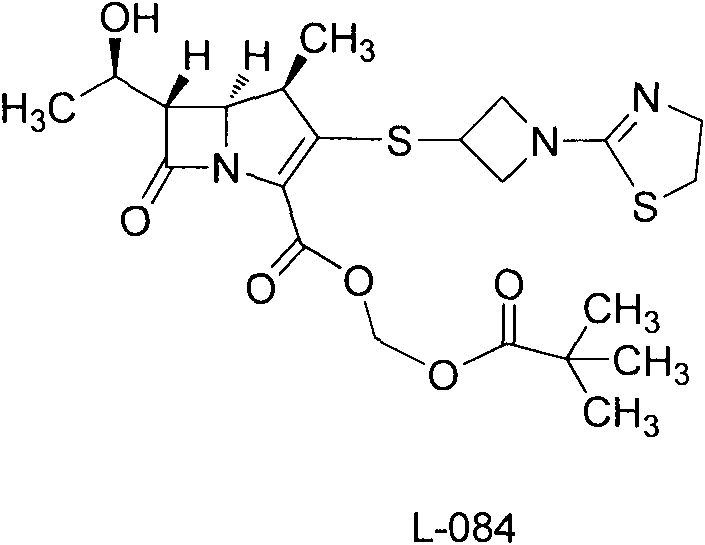
Crystal of intermediate of tebipenem pivoxil and preparation method thereof
A technology for tipipenem and intermediates, applied in the field of crystallization and preparation of pharmaceutical intermediates, can solve the problems of low purity and unfavorable industrialization, and achieve the effects of high purity, good stability and easy crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
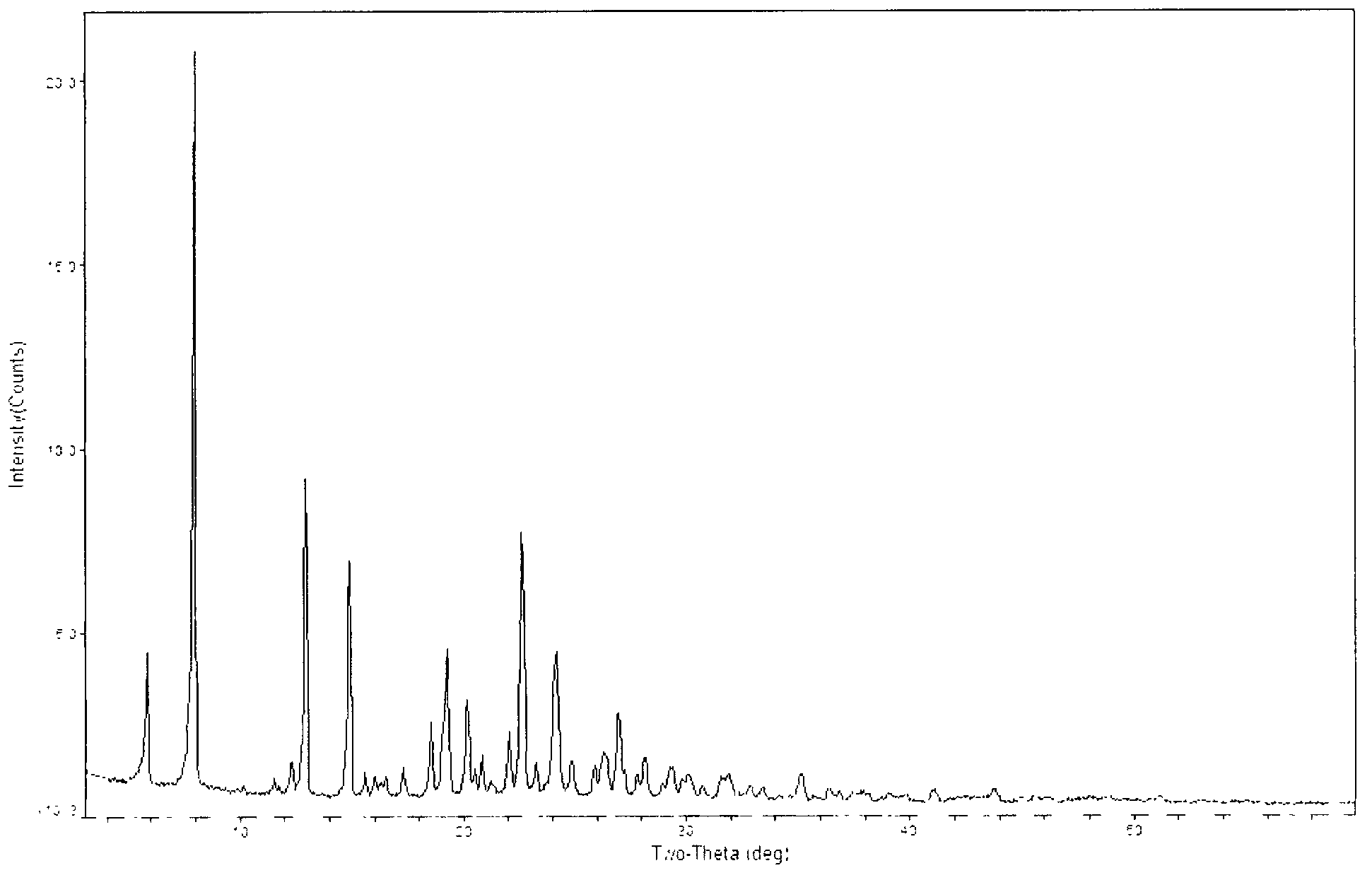
[0031] 105 g of 1-(4,5-dihydro-2-thiazolyl)azetidine-3-thiol hydrochloride, [4R-[4a, 5b, 6b(R*)]]-3-di Phenylphosphoryloxy-6-(1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid p-nitro Benzyl ester (MAP) 297g was mixed in 1700ml of acetonitrile, cooled and stirred, DIEA was added dropwise at -10°C, the drop was completed, and the reaction was kept for 2h. The reaction endpoint was monitored by HPLC. Add 1000ml of water and stir for 0.5h. Suction filtration, washing with 500 ml of acetonitrile-water, and vacuum drying at 50° C. for 2 h gave 258 g of light yellow solid crystals with a yield of 99.1%. That is the A crystal form.
[0032] The obtained crystals were analyzed by X-ray diffraction test, and the test conditions were as follows: Bruker D8 ADVANCE instrument was used to measure, CuKa40Kv40mA was used as light source, step size was 0.02°, scanning speed: 8° / min, scanning range: 3°~80°, room temperature. Powder X-ray diffraction analysis re...
Embodiment 2
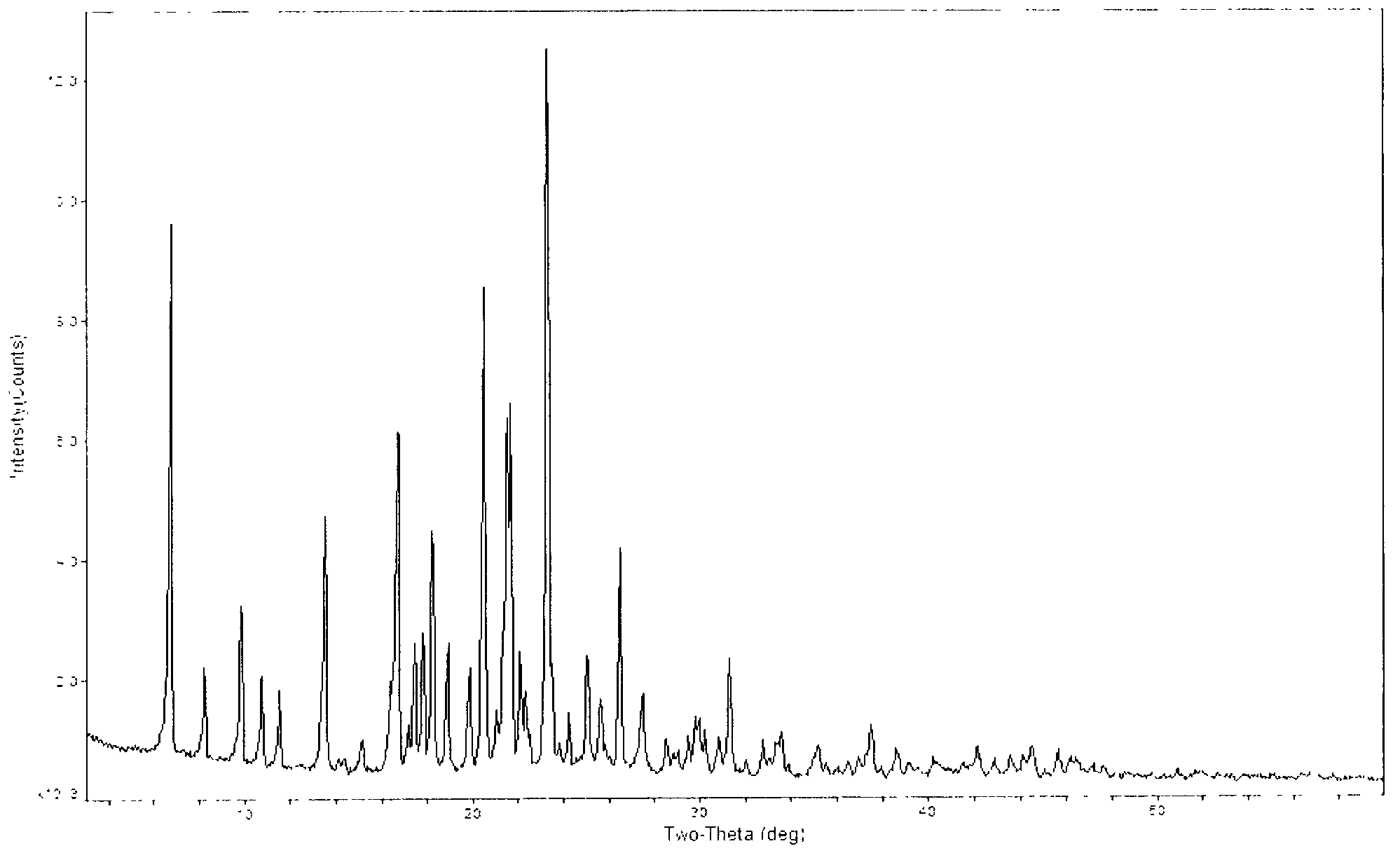
[0036] The solid (50g) obtained in Example 1 was added to 500ml of ethanol, heated to reflux for 30min, cooled to room temperature, stirred at -5 to 5°C for 2h, filtered with suction, washed with 50ml of cold ethanol, and dried under reduced pressure at 45°C to obtain a The white solid was 46 g, the yield was 92%, and the HPLC purity was 99.98%, which was crystal form B.
[0037] The obtained crystals were tested and analyzed by X-ray diffraction. The test conditions were as follows: Bruker D8 ADVANCE instrument was used to measure, with CuKa 40Kv40mA as the light source, step size 0.02°, scanning speed: 8° / min, scanning range: 3°~80°, room temperature . Powder X-ray diffraction analysis results are shown in Table 2 and attached figure 2 .
[0038] Table 2 Powder X-ray Diffraction Data of Type B Crystal
[0039]
[0040]
Embodiment 3
[0042] The solid (20 g) obtained in Example 1 was added to 200 ml of methanol, heated to reflux for 30 min, cooled to room temperature, stirred at -10 to 0 °C for 2 h, filtered with suction, washed with 10 ml of cold methanol, and dried under reduced pressure at 45 °C to obtain a The white solid was 15.2 g, the yield was 76%, and the HPLC purity was 99.94%, which was crystal form B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com