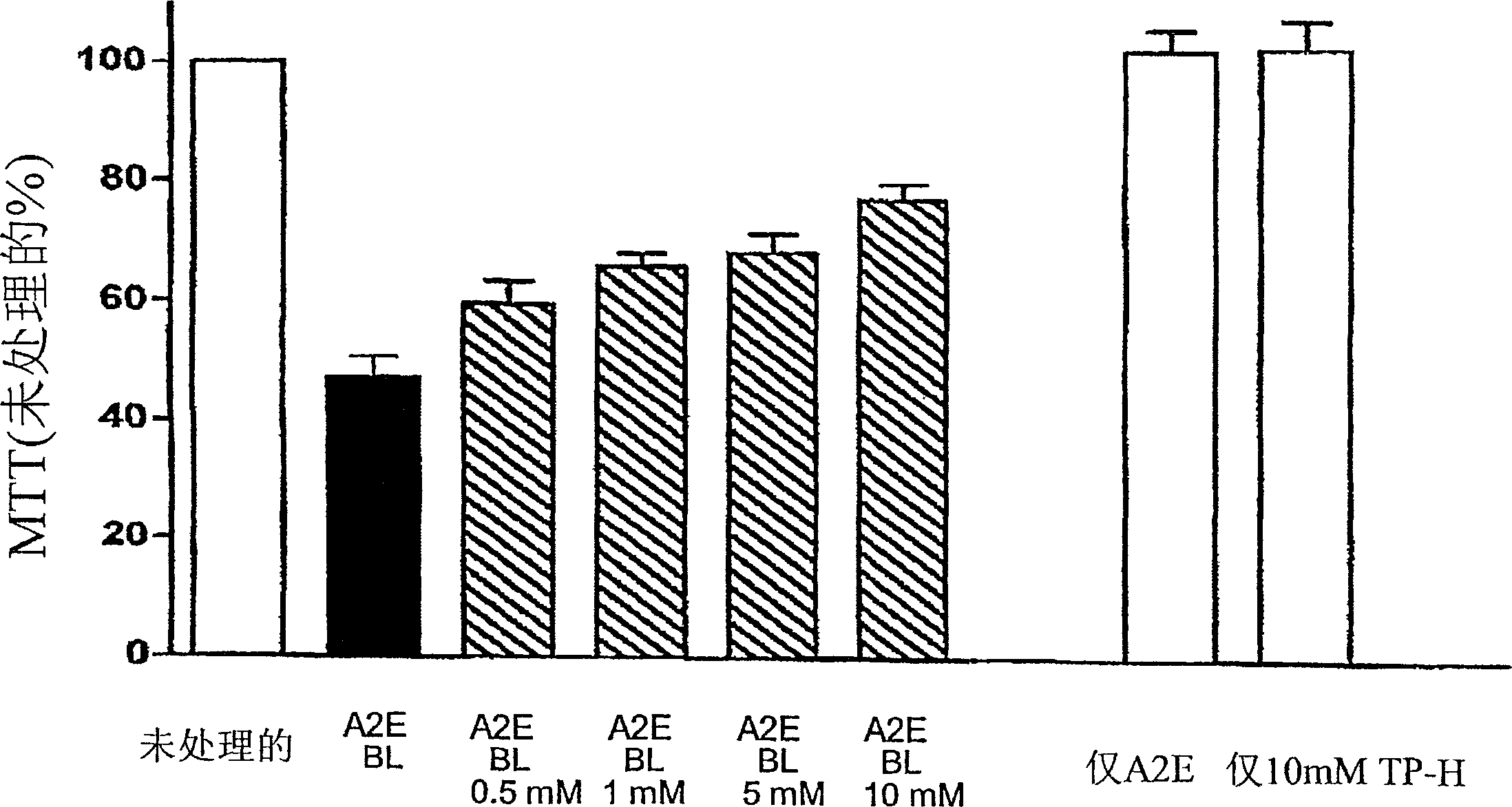Amelioration of macular degeneration and other ophthalmic diseases
A macular degeneration and disease technology, applied in skin diseases, sensory diseases, cardiovascular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: Therapeutic effect of Tempol-H on photochemical retinal damage in a rabbit model
[0075] The ability of light to produce retinal damage far below the level at which it can produce thermal damage is referred to as photochemical retinal damage. It is generally believed that the mechanism of action of photochemical retinal damage is the generation of free radicals induced by light. The ability of common light sources used in clinical ophthalmology to produce photochemical retinal damage is now well appreciated. Manipulating microscopes used in ophthalmic surgery have demonstrated the ability to produce photochemical damage to the retina. This observation has been used to create a model in the rabbit eye to test the defense of various agents against actinic retinal damage. It has been determined that optically visible retinal damage is detectable after only 2.5 minutes of exposure to an operating microscope. The protocol set out below can be used to determine...
Embodiment 2
[0079] Example 2: Evaluation of Tempol-H Incorporation into Rabbit Retinal Tissue Following Intravitreal Injection
[0080] In order to evaluate the effectiveness of tempol-H in preventing AMD and other retinal disorders, it is necessary to establish that the drug has incorporated into retinal tissue. The protocol set out in this example employs scintillation techniques to determine the amount and duration of tempol-H incorporation into retinal tissue following intravitreal injection into the rabbit. Because the large eyes of rabbits provide an appropriate template for therapeutic administration, New Zealand white rabbits were characterized in detail in experiments involving intravitreal injections (Hosseini et al., 2003, Lasers Surg. Med. 32:265-270). In addition, New Zealand white rabbits have been used extensively in experiments evaluating drug uptake into retinal tissue by scintillation techniques (Ahmed et al., 1987, J. Pharm. Sci. 76:583-586).
[0081] Materials and Met...
Embodiment 3
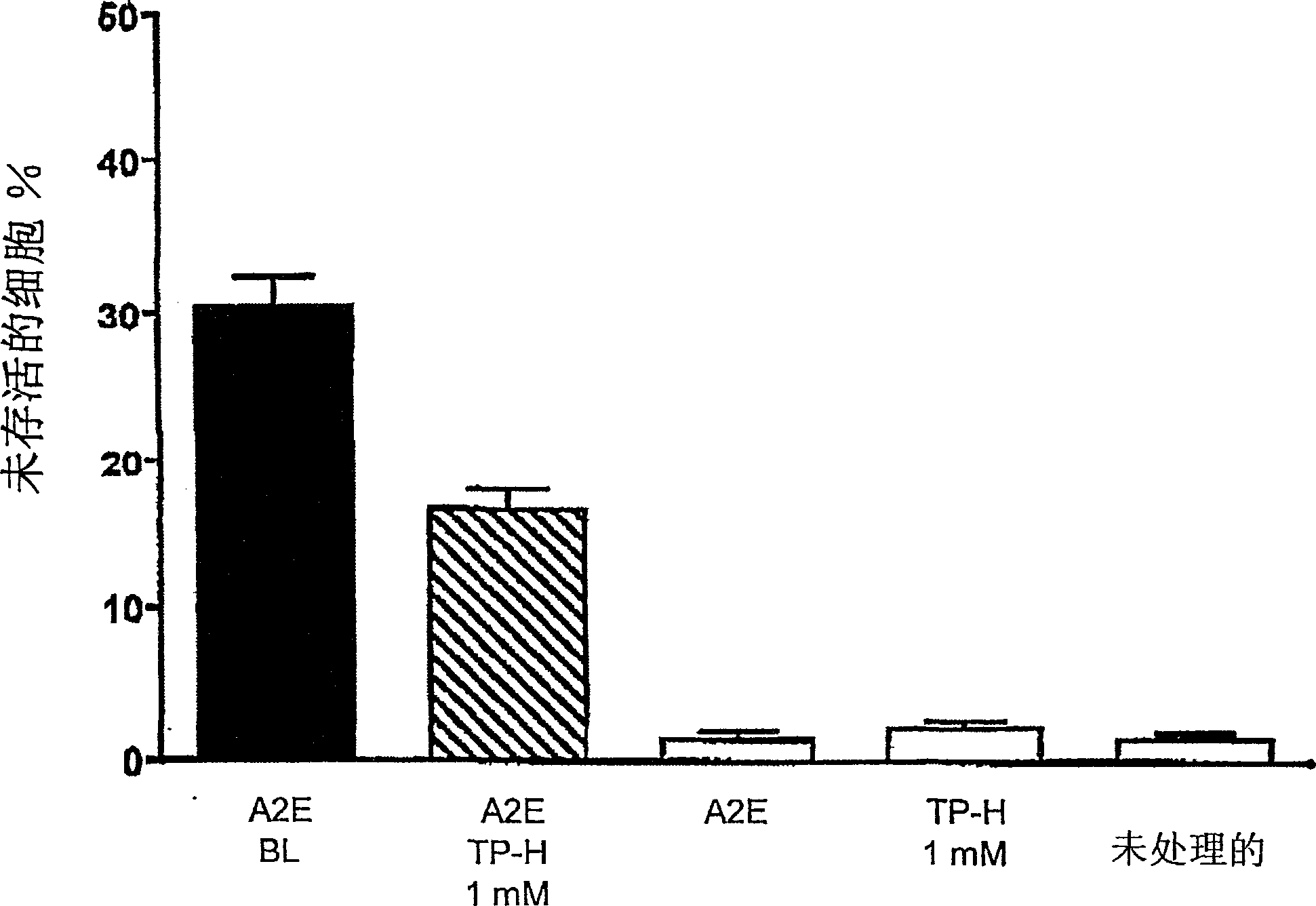
[0088] Example 3: Efficiency of Tempol-H in Protecting Photooxidative Processes in Retinal Pigment Epithelial Cells (RPE)
[0089] background:
[0090] Although the etiology of atrophic age-related macular degeneration (AMD) is unclear, it is generally accepted that AMD begins with the death of retinal pigment epithelium, degeneration of photoreceptor cells, and the consequent visual impairment lost. There is increasing evidence linking lipofuscin accumulation in the RPE to the death of these cells. For example, not only the level of lipofuscin in RPE cells below the macula is the highest, but the measurement of lipofuscin in RPE by automatic fundus fluorescence also demonstrates that at the site of previous fluorescence enhancement, the area of RPE atrophy will expand accordingly. Studies on the relationship between RPE lipofuscin and RPE cell death have shown that the component of RPE lipofuscin, the bisretinoid luminophore A2E, can disrupt cell membranes and mediate blu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com