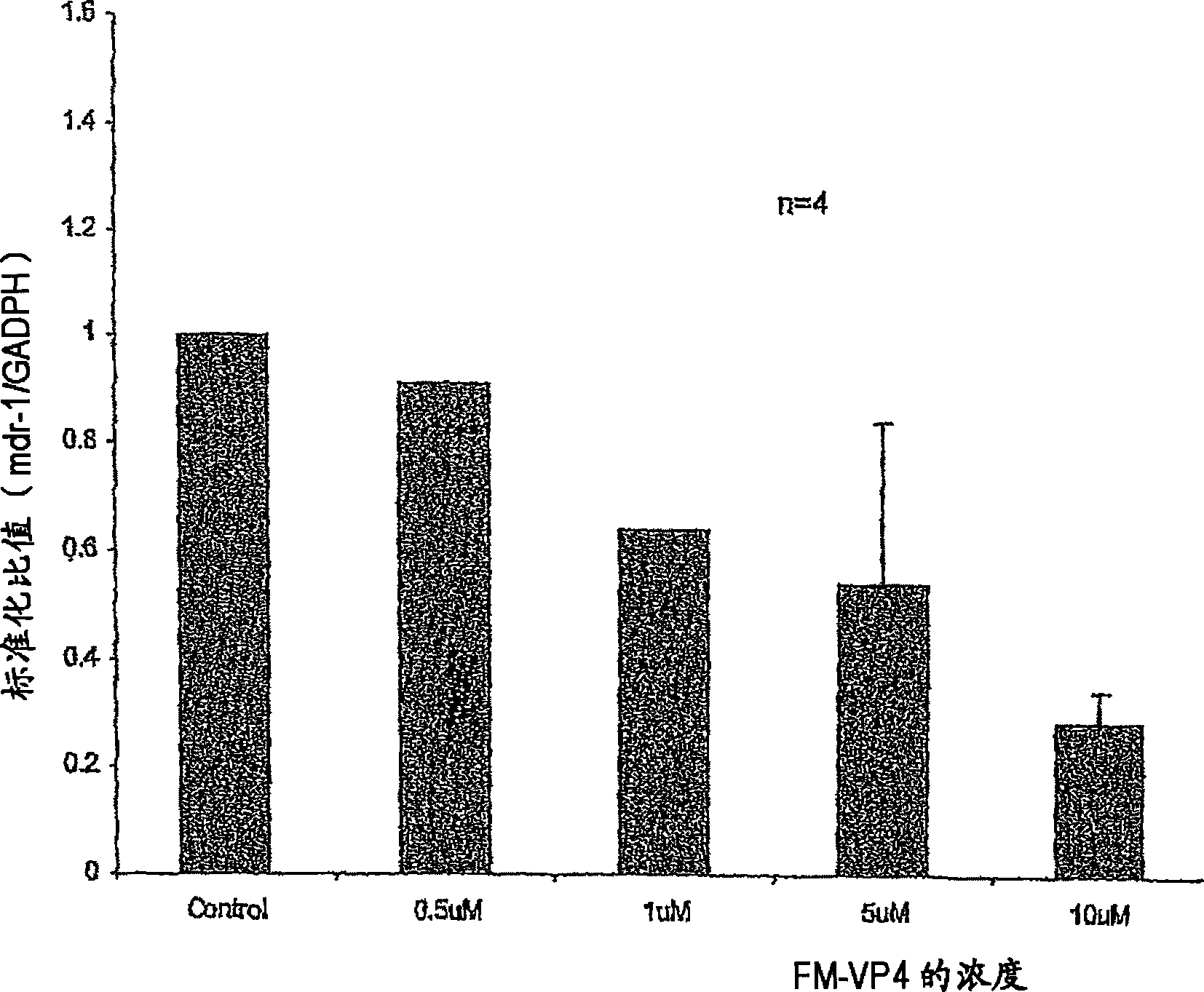
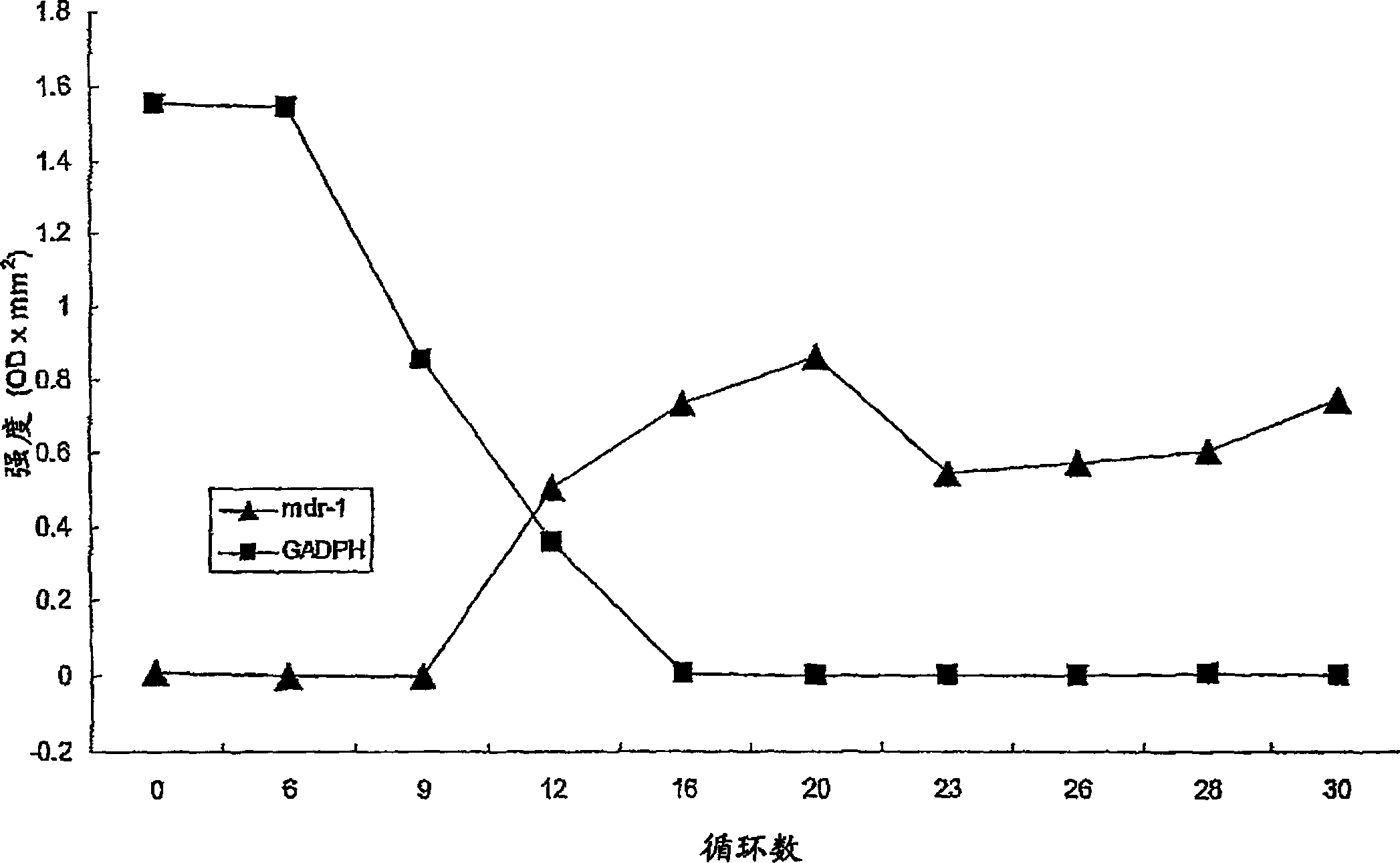
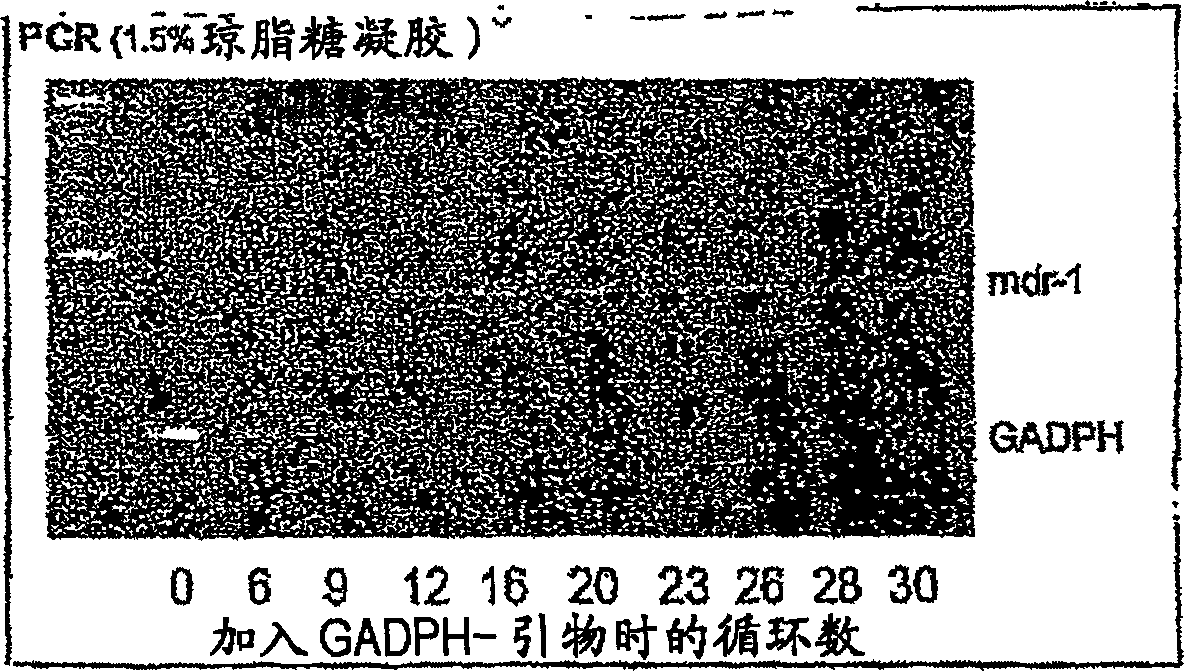
Method of inhibiting the expression of a multi-drug resistance genes and inhibiting the production of proteins resulting from the expression of such genes thereby enhancing the effectiveness of chemot
A gene expression and drug resistance technology, applied in the field of cancer treatment, can solve the problems of clinical reversal limitation of resistance, worsening patient prognosis, and binding chemotherapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0225] Example 1 - A Cholesterol Absorption Inhibitor as described herein: Preparation of ascorbyl stanolyl phosphate referred to herein as "FM-VP4";
[0226] Step 1: Protect Ascorbic Acid
[0227] Fuming sulfuric acid (24%, 8.3g) was added dropwise to acetone (50ml). Ascorbic acid (12 g) was introduced into the mixture at 0°C, and the reaction mixture was stirred at 0°C for 6 hours. The resulting crystals were filtered with suction, and the filter cake was pressed dry and washed with acetone (30 ml). The product, 5,6-isopropylidene ascorbic acid (14 g) was obtained.
[0228] Step 2: Linking to Phytostanols
[0229] At 0°C, a solution of phytostanol mixture (24g) (camesteranol: 36.4%; sitostanol: 62.3%) in toluene (500ml) and pyridine (25ml) was added dropwise to phosphorus oxychloride ( 9ml) in a mixture in toluene (200mol). The mixture was stirred at room temperature for 3 hours. Pyridine hydrochloride was filtered off, and the mother liquor was concentrated to recover...
Embodiment 2
[0232] Example 2: - a kind of cholesterol absorption inhibitor described in the text: preparation of disodium ascorbyl phosphate of dehydroepiandrosterone
[0233] Acetone (150 ml) and L-ascorbic acid (50 g) were added to a dry round bottom flask at 0°C. Acetyl chloride (7.5ml) was added dropwise via addition funnel over 10 minutes. The reaction mixture was stirred at 0 °C for 24 hours. The precipitate was filtered off and washed with acetone (3 x 20ml). The white product, 5,6-isopropylidene ascorbic acid, was dried under vacuum for 1.5 hours to obtain a dry powder (52 g), yield 85%.
[0234] A dry three-neck round bottom flask was equipped with a stir bar, argon inlet and addition funnel. A solution of dehydroepiandrosterone (1.73 g, 6 mmol) in anhydrous THF (15 ml) and pyridine (2.4 ml) was added dropwise to anhydrous THF (12 ml) and POCl at 0 °C over 10 minutes 3 (0.7ml, 7.5mmol) in a mixture. A white precipitate formed immediately. The suspension was stirred for 40 m...
Embodiment 3
[0238] Example 3 - Another cholesterol absorption inhibitor described herein: Synthesis of disodium ascorbyl phosphate of 5α-androstane-3β-ol-17-one
[0239] To a dry round bottom flask was added 5α-androstane-3β-ol-17-one (1.0 g, 3.4 mmol), THF (8.6 ml) and pyridine (1.38 ml). The mixture was stirred at room temperature until a clear solution was obtained. Into another dry round bottom flask was added THF (6.9ml) and POCl 3 (0.4ml, 4.25mmol), stirred at 0°C for 5 minutes. To this mixture was added dropwise the 5α-androstane-3β-ol-17-one solution prepared above under an argon atmosphere over 10 minutes. After the addition was complete, the white suspension was stirred at 0°C for 35 minutes and at room temperature for 2 hours. The reaction was stopped and the white suspension was used for the coupling reaction without filtration.
[0240] 5,6-Isopropylidene ascorbic acid (2.0 g, 9.52 mmol) was dissolved in pyridine (1.71 ml) and THF (17 ml). The round bottom flask containi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com