Method of refining salt by membrane filtration
A technology for refined brine and membrane filtration, applied in chemical instruments and methods, membrane technology, semi-permeable membrane separation, etc., can solve problems such as large quality impact, unstable brine quality, and excessive brine, and reduce the number of backwash cleaning , The effect of ensuring high permeation flux and reducing processing load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
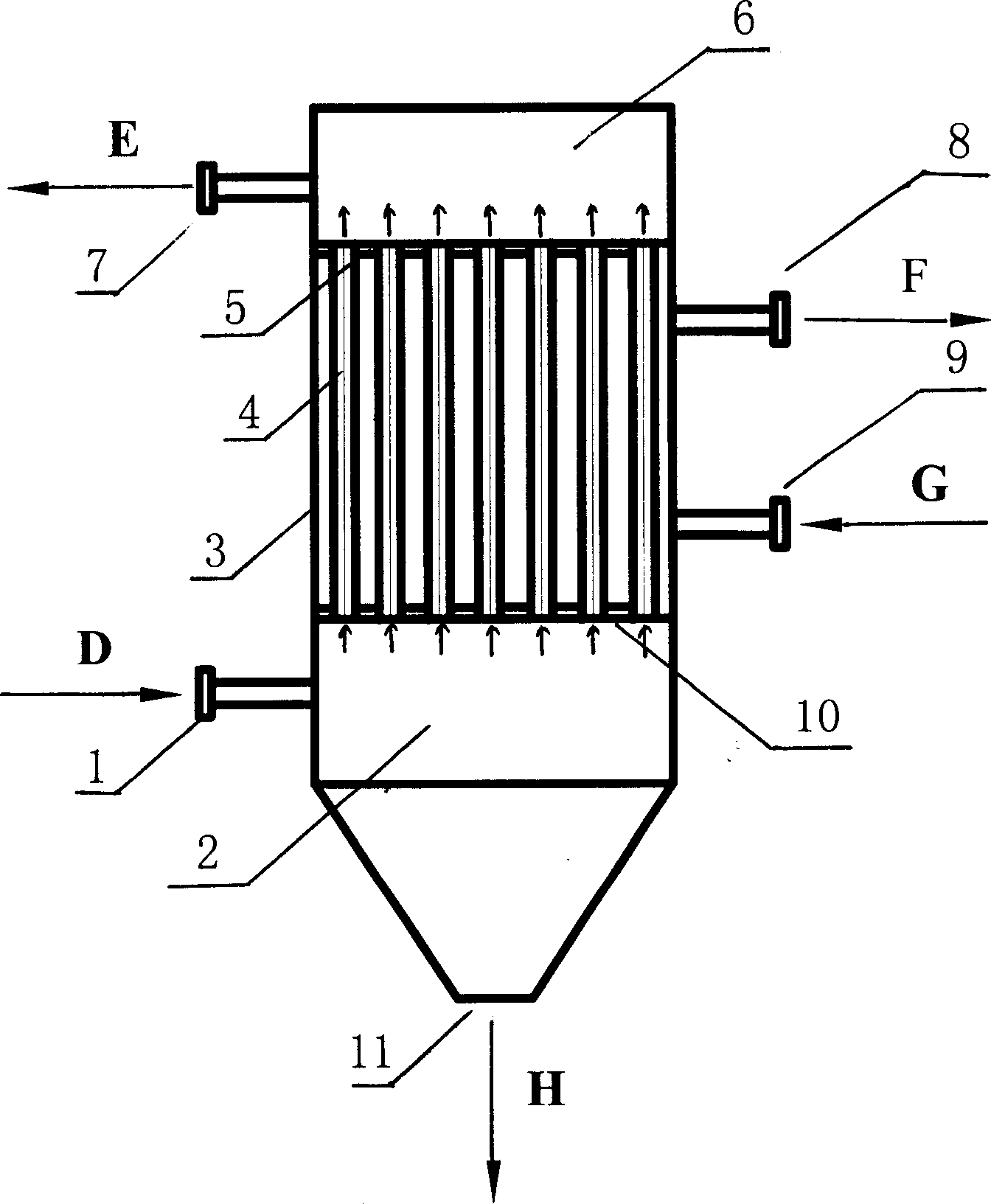
[0036] Below in conjunction with figure, the present invention is described in detail
[0037] A) Refined brine and impurity-rich brine from the evaporation section enter the brine storage tank, and enter the brine tank through the brine pump, and the brine dissolves the original salt to become saturated coarse brine, which overflows from the upper part of the brine tank to the reaction tank ;
[0038] B) metering into the reaction tank the refining agent NaOH, NaOH 2 CO 3 , BaCl 2 and other chemicals make the Ca in brine 2+ , Mg 2+ , SO 4 2- Wait for the reaction to generate Mg(OH) 2 , CaCO 3 with BaSO 4 and other solid substances, in which NaOH and Na 2 CO 3 Adding concentrations were greater than Ca 2+ with Mg 2+ Concentration 1-5%, BaCl 2 The added concentration is less than SO 4 2- Concentration 1-5%;
[0039] C) The above-mentioned brine enters the preprocessor, and the coagulation aiding agent sodium polyacrylate, polyacrylamide or causticized starch is...
Embodiment 2
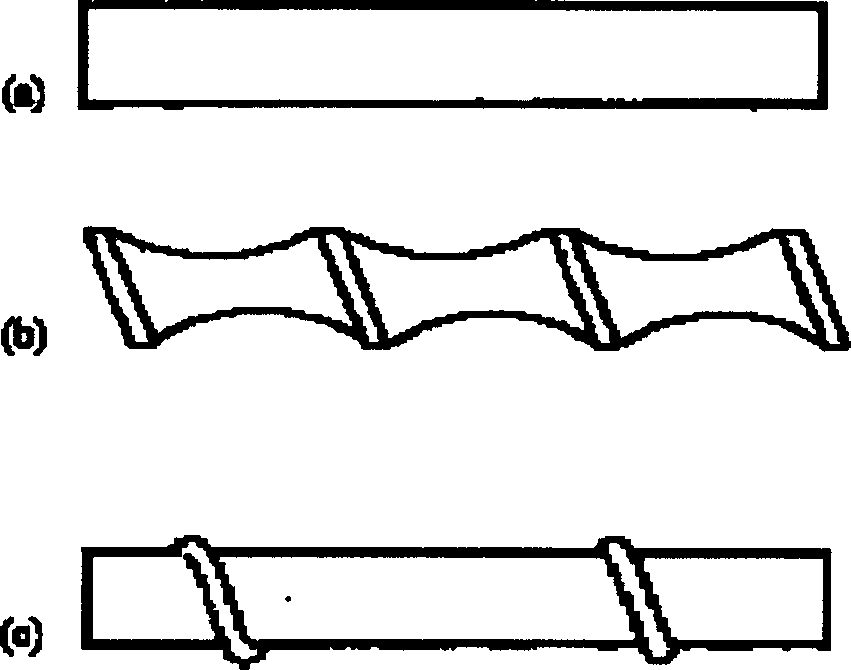
[0042] After salting, the calcium content in the crude brine is 754.2mg / L, the magnesium content is 83.21mg / L, the sulfate content is 7.6g / L, and the SS content is 3589.33mg / L. Send this saturated brine into the reaction tank, add sodium hydroxide 0.4g / L, sodium carbonate 1.6g / L and barium chloride 12.5g / L to react, after the reaction is completed, enter the preprocessor, add polyacrylamide 50mg / L l and hydrogen peroxide 30mg / l, after adsorption and co-precipitation, pumped into the inorganic membrane cross-flow filter, using a ceramic membrane with a pore size of 0.02μm, 19 channels, the inner diameter of the channel is 4mm (no turbulence promoter is installed in the channel), cross-flow Speed 1m / s, operating pressure 0.4MPa, membrane permeation flux 0.5m 3 m -2 h -1 , the experiment was carried out for 5h without backflushing the membrane. The filtered brine was measured by ICP (plasma emission spectrometry), and calcium, magnesium and SS were not detected, and the sulf...
Embodiment 3
[0044] The same saturated brine of Example 1 is sent into the reaction tank, and sodium hydroxide 0.4g / L, sodium carbonate 1.6g / L and barium chloride 12.5g / L are added to react, after the reaction is completed, enter the preconditioner, add poly Sodium acrylate 60mg / l and sodium hypochlorite 40mg / l, after adsorption and co-precipitation, are pumped into the inorganic membrane cross-flow filter, using a ceramic membrane with a pore size of 0.02μm, 7 channels, the inner diameter of the channel is 6mm, and the cross-flow velocity is 2m / s, operating pressure 0.3MPa, membrane permeation flux 0.6m 3 m -2 h -1 , The experiment was carried out for 10h without backflushing the membrane. The filtered brine was measured by ICP (plasma emission spectrometry), and calcium, magnesium, and SS were not detected, and the sulfate content was 5.0mg / L, which fully met the standard of high-quality refined brine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com