A preparation method of compound rifampicin preparation
A rifampicin and compound technology, which is applied in the field of preparation of compound rifampicin preparations, can solve the problems such as the decline in the quality of FDC preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation and in vitro drug release characteristics of isoniazid enteric-coated granules:
[0038] 1. Preparation of isoniazid enteric-coated granules
[0039] The prescription composition of isoniazid granules: 75g of isoniazid, 6g of starch, and an appropriate amount of 5% povidone (K30) solution.
[0040] Granulation: Weigh each raw material and auxiliary material according to the above prescription, pass through a 100-mesh sieve, mix evenly, granulate with 5% povidone (K30) solution, dry in an oven at 60°C, sieve, granulate, and set aside.
[0041] Preparation of enteric coating solution: take 5 g of hydroxypropylmethyl phthalate, add 100 ml of acetone / ethanol solution (3 / 7) to dissolve, add 0.5 g of diethyl phthalate, and dissolve evenly.
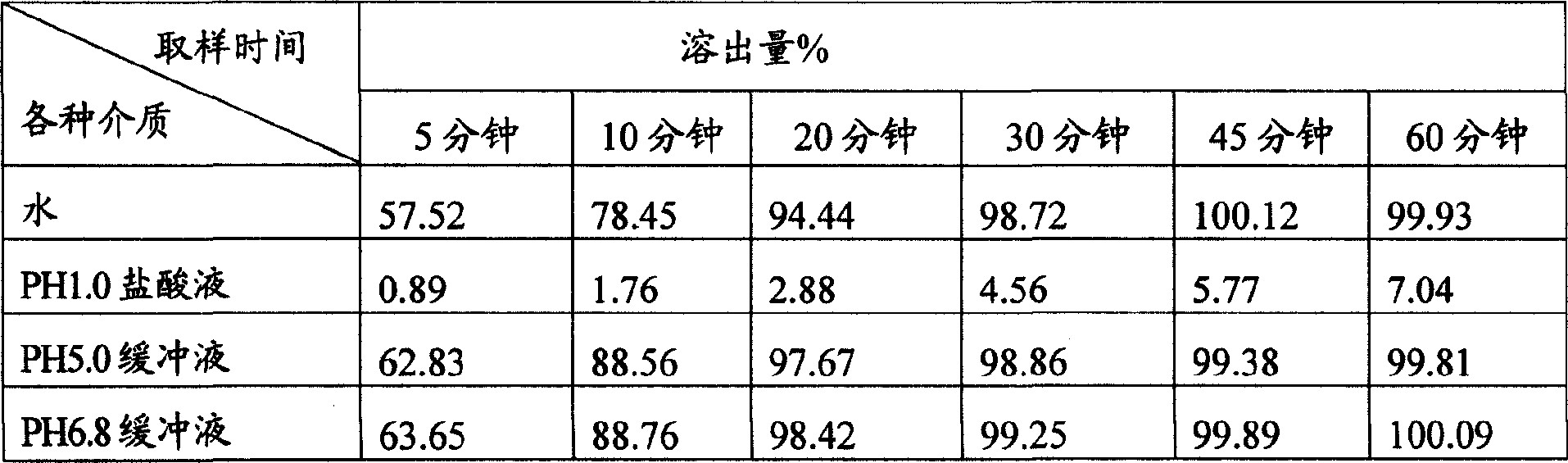
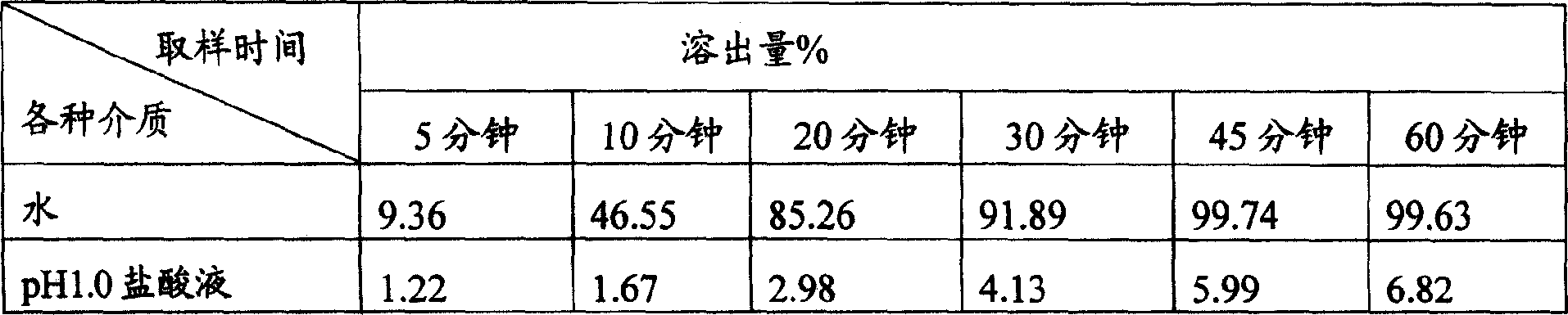
[0042] Preparation of enteric-coated granules: Put the prepared granules in the coating pan, roll the coating pan, spray the enteric coating solution while blowing hot air, wrap the granules until the weight of the ...
Embodiment 2
[0048] Example 2 Preparation and in vitro drug release characteristics of rifampicin enteric-coated granules
[0049] 1. Preparation of rifampicin enteric-coated granules
[0050] The prescription composition of rifampicin granules: rifampicin 150g, microcrystalline cellulose 45g, vitamin C 3g, sodium carboxymethyl starch 2g.
[0051] Granulation: Weigh each raw material and auxiliary material according to the above prescription, pass through an 80-mesh sieve, mix evenly, dry granulate, granulate, and set aside.
[0052] Preparation of film coating solution: take 3 g of hydroxypropyl methylcellulose and 3 g of polyethylene glycol 4000, and dissolve them evenly with 70% ethanol solution.
[0053] Preparation of film coating: put the prepared granules in the coating pan, roll the coating pan, spray the film coating solution while blowing hot air, wrap until the granules increase in weight to about 3%, take them out and dry them in an oven at 50°C for 1 Hour.
[0054] Preparat...
Embodiment 3
[0062] Example 3 Preparation and in vitro drug release characteristics of rifampicin gastric soluble granules
[0063] 1. Preparation of rifampicin gastric soluble granules
[0064] The prescription composition of rifampicin granules: rifampicin 150g, microcrystalline cellulose 30g, vitamin C 3g, low-substituted hydroxypropyl cellulose 10g.
[0065] Granulation: Weigh each raw material and auxiliary material according to the above prescription, pass through an 80-mesh sieve, mix evenly, dry granulate, granulate, and set aside.
[0066] Preparation of gastric soluble coating solution: take 9 g of gastric soluble film coating premix, add 100 ml of 50% ethanol solution and dissolve evenly.
[0067] Preparation of gastric soluble granules: put the prepared granules in the coating pan, roll the coating pan, spray the gastric soluble coating liquid while blowing hot air, wrap until the weight of the granules increases to about 4%, take them out and dry them in an oven at 50°C Take...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com