Novel indazole derivative
A technology of indazole and compound, applied in the field of novel indazole derivatives or their salts, to achieve excellent Rho kinase inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0300] The production method of the synthetic intermediate of the compound of the present invention will be described in detail in the following Examples [Production Items].
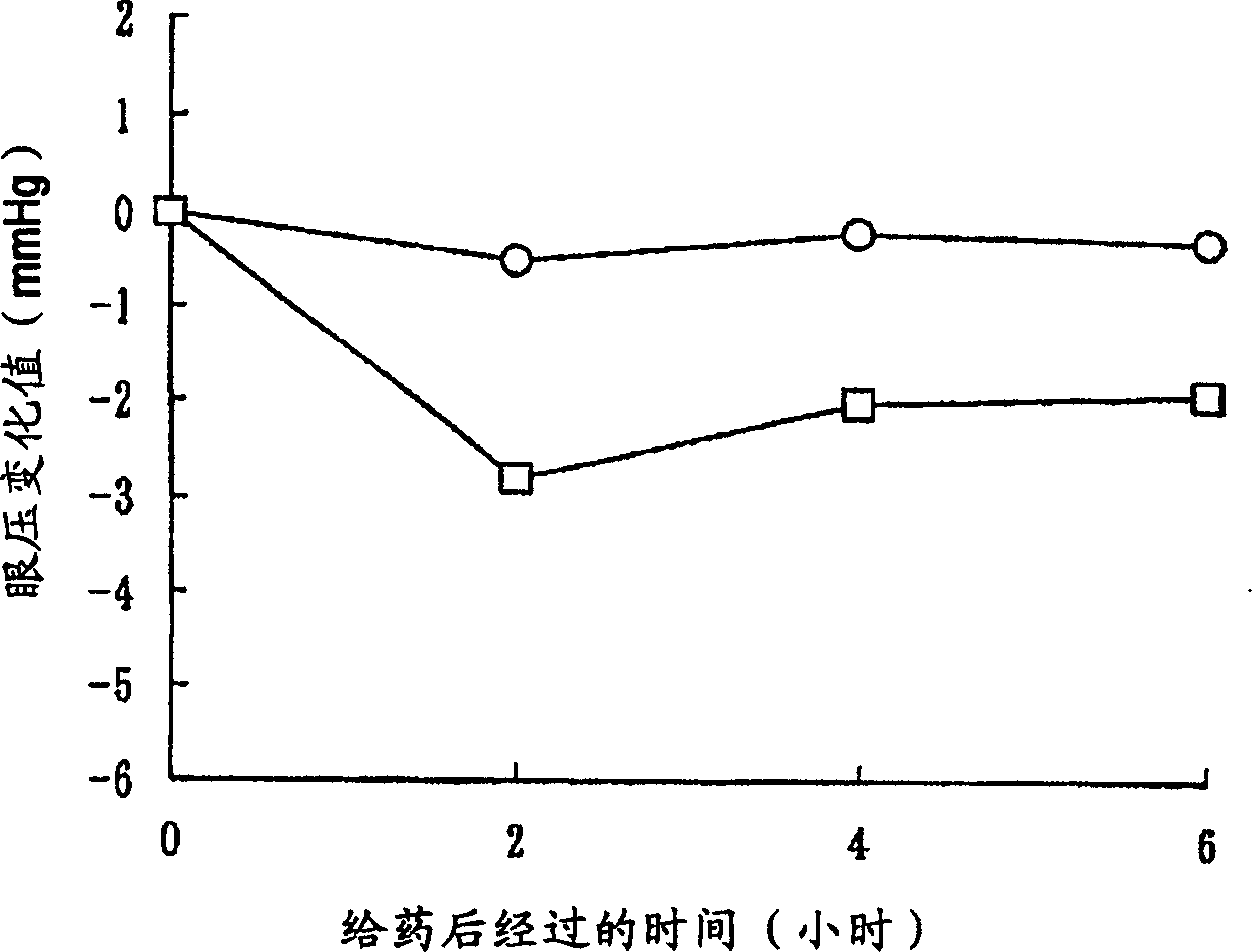
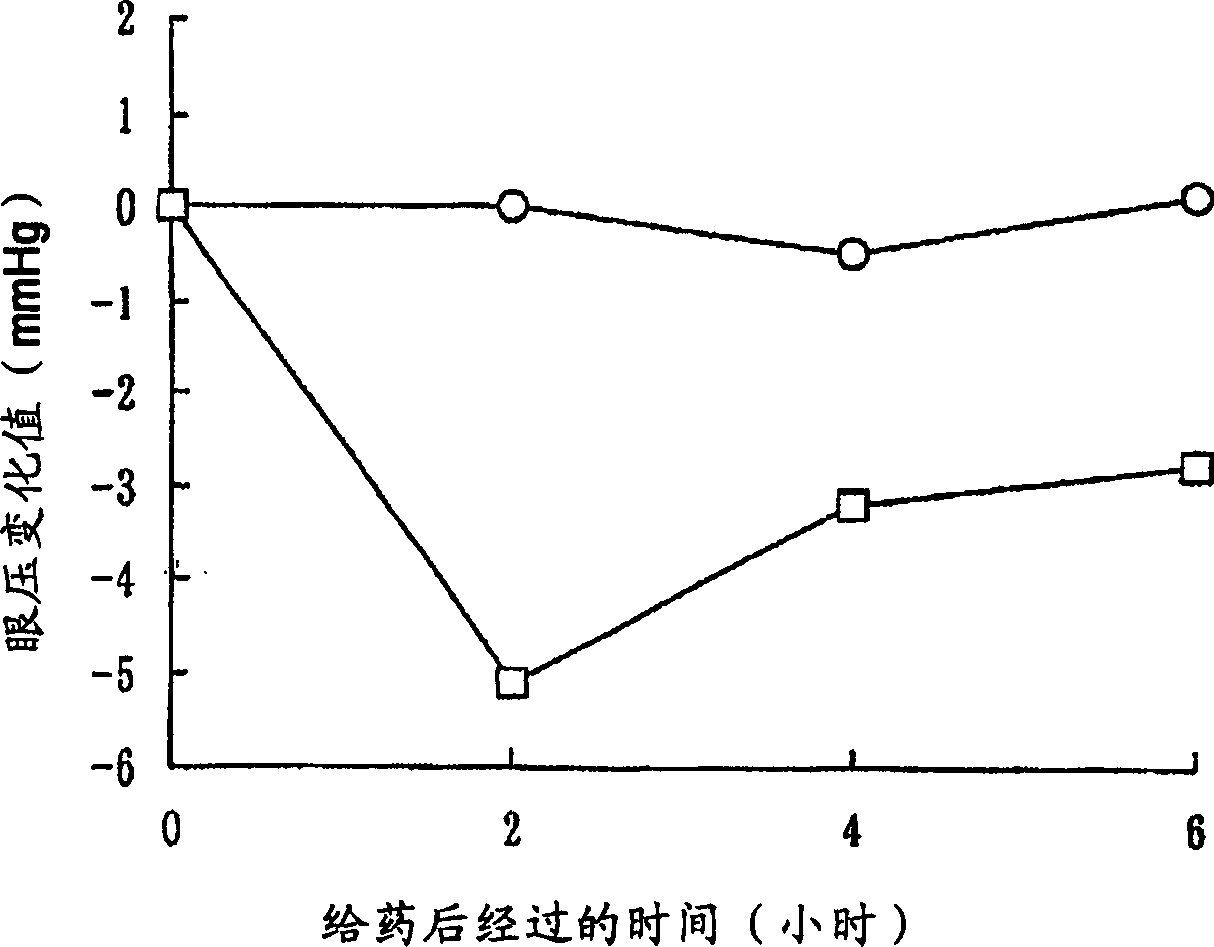
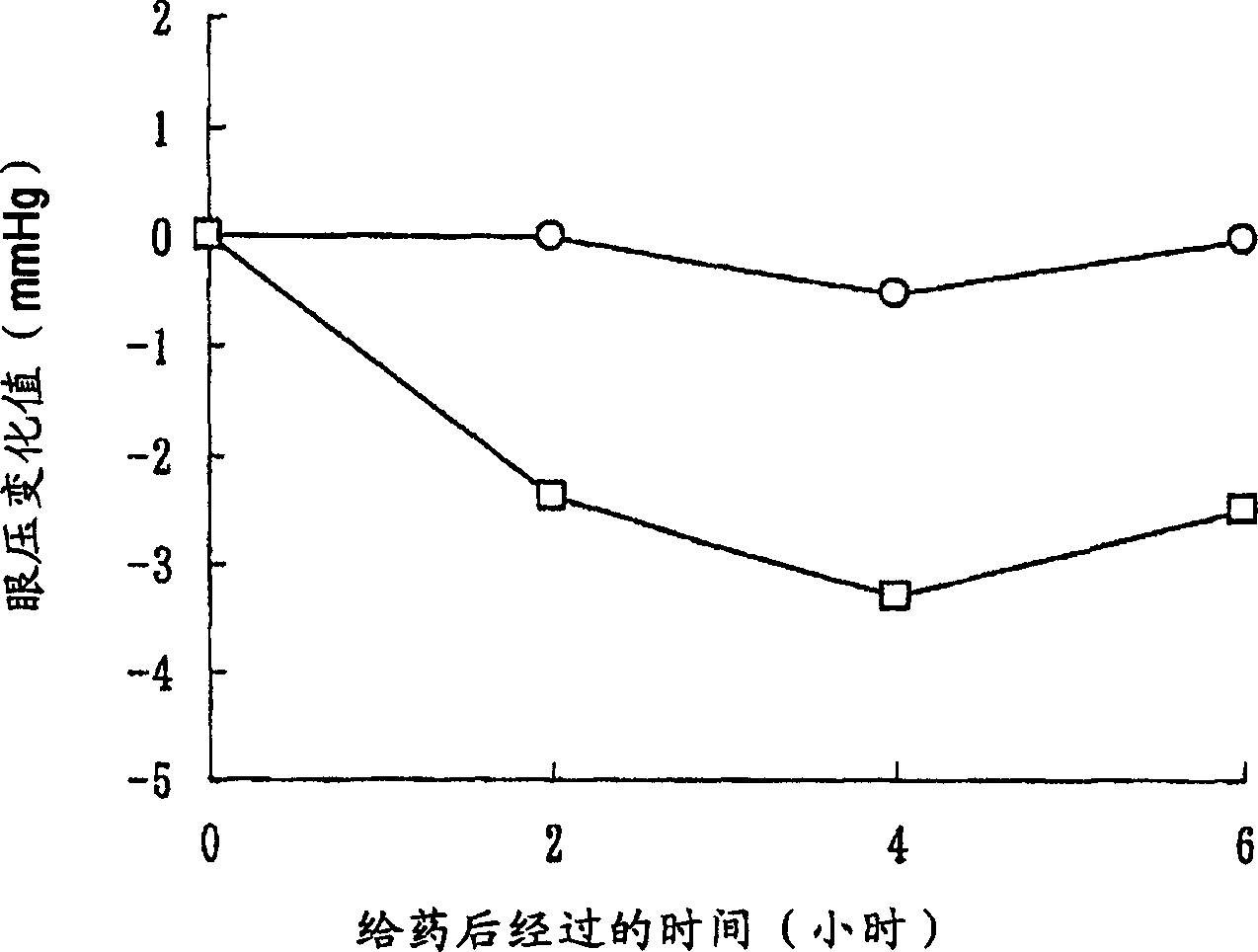
[0301] In order to show the usefulness of the compounds of the invention, the Rho kinase inhibitory effect of the compounds of the invention was evaluated. Its detailed description is shown in the following example [pharmacological test items (Rho kinase inhibitory activity evaluation test)], based on Journal of biological chemistry issued in 1999, volume 274, page 32418 [J.Biol.Chem., 274, 32418 ( 1999)], the method of Beiyuan et al. and the method of commercially available active type ROCK II [upstatebiotechnology, Catalog No. 14-338, (5Unit / 50ml] attached instruction manual), the Rho kinase inhibitory activity of the compound of the present invention was carried out. Evaluation. As a result, it was found that the compound of the present invention has an excellent Rho kinase inhibitory effect and is ve...
reference example 1
[0315] Synthesis of 1-bromo-4-(1-cyano-1-methylethyl)benzene (reference compound 1-1)
[0316]
[0317] In a solution containing 100g (510mmol) of 4-bromophenylacetonitrile in 1500ml of N,N-dimethylformamide, under an argon flow, 45g (1100mmol) of sodium hydride (60 % mineral oil dispersion). Then, 95 ml (1500 mmol) of iodomethane was added dropwise with stirring at 0°C, and stirred at 10°C for 1 hour.
[0318] After the reaction was completed, the reaction solution was slowly poured into 900 g of saturated ammonium chloride aqueous solution, and 500 ml of water was added, followed by extraction with 2000 ml of ethyl acetate. The organic layer was washed with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 110 g of the title compound as a dark brown oil. (Yield: 96%)
[0319] Rf value: 0.78 (n-hexane:ethyl acetate=1:1 (V / V)).
[0320] MS (CI, m / z): 224, 226 (M + +1).
[0321] 1 H-NMR (CDCl 3 ,...
reference example 2
[0352] Synthesis of 4-(1-aminocarbonyl-1-methylethyl)-1-bromobenzene (reference compound 2-1)
[0353]
[0354] In 1000ml of toluene solution containing 100g (450mmol) of 1-bromo-4-(1-cyano-1-methylethyl)benzene (reference compound 1-1), under argon flow, while stirring, at room temperature 250 g (1800 mmol) of potassium trimethylsiliconate (purity: 90%) was added, and stirred for 4.5 hours under heating and reflux.
[0355] After the reaction, the reaction solution was cooled to room temperature, and 500 ml of water was added dropwise. After stirring the mixed solution at room temperature for 25 minutes, the resulting solid was filtered. The above solid was washed with 400 ml of water to obtain 99 g of the title compound as a white powder. (Yield: 92%)
[0356] Melting point: 139-141°C.
[0357] Rf value: 0.23 (n-hexane:ethyl acetate=1:1 (V / V)).
[0358] MS (CI, m / z): 242, 244 (M + +1).
[0359] 1 H-NMR (CDCl 3 , δppm): 1.56 (s, 6H), 5.18 (brs, 1H), 5.52 (brs, 1H)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com