Sequence selective pyrrole and imidazole polyamide metallocomplexes
A technology for imidazole polyamides and complexes, which is applied in the directions of active ingredients of heterocyclic compounds, drug combinations, organic active ingredients, etc., can solve the problem of lack of sequence selectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
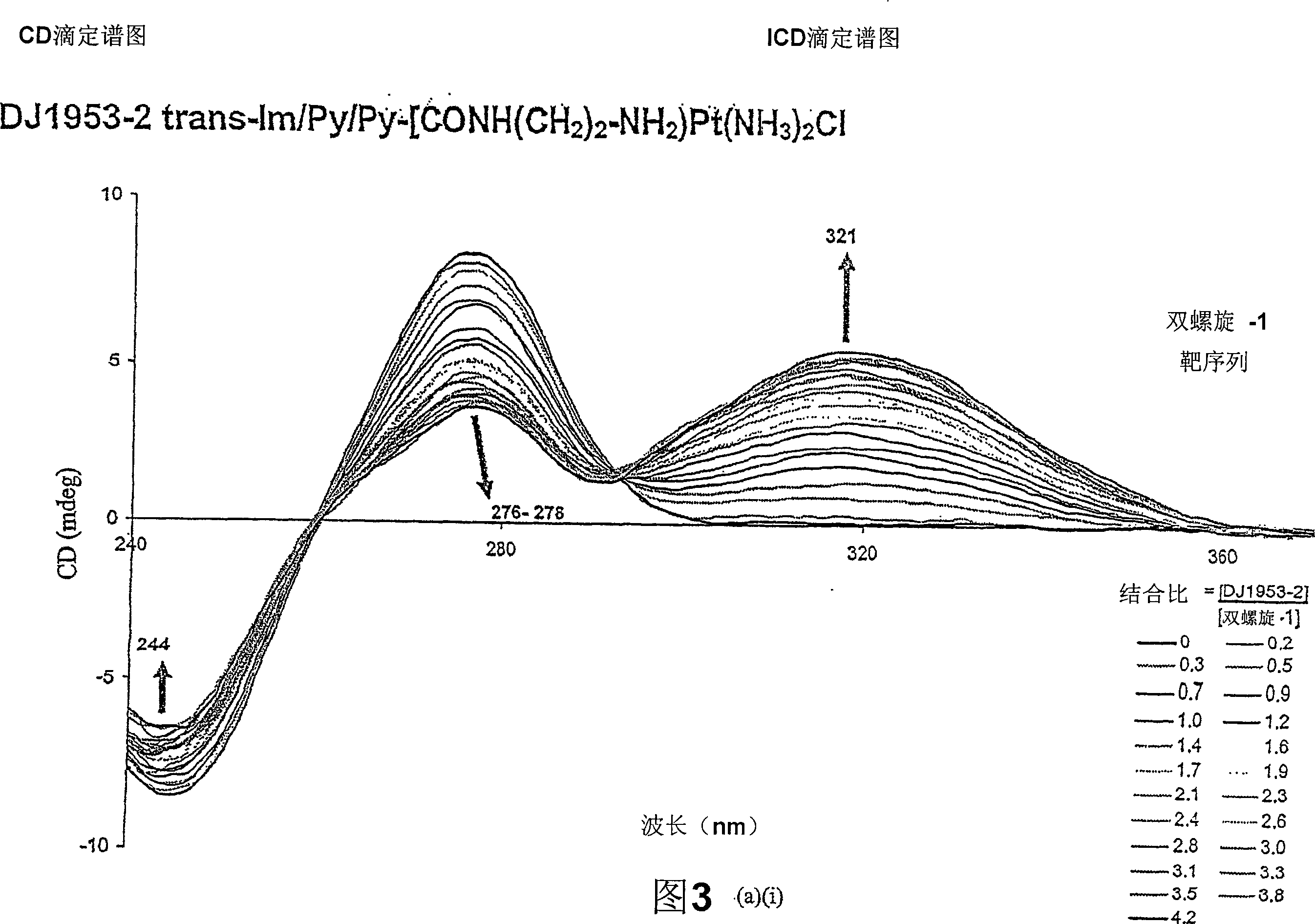
Image
Examples
preparation example Construction
[0301] Compound preparation
[0302] The compounds of the present invention are suitable for preparation in a modular or stepwise manner. Gradual synthesis allows people to change the number and composition of the component polyamides, linkers and metal composites in a controlled manner. For example, the length and composition of the polyamide chain can be selected to target a specific nucleotide sequence. The modular compounds of structural formulas (1), (3), (4) and (5) can be connected with other modular compounds, or connected to another metal compound, to generate structural formulas (1), (3) or (5) Compounds with the required number of pyrrole-imidazole polyamides capable of selectively targeting specific polynucleotide sequences. The compounds of the present invention can be prepared, for example, the compounds of structural formula (1), (3), (4) and (5), which can be targeted to contain about 2, 3, 4, 5, 6, 7, 8, 9 or about The polynucleotide sequence of the selected core ...
Embodiment 1
[0355] Synthesis of Im / Py / Py
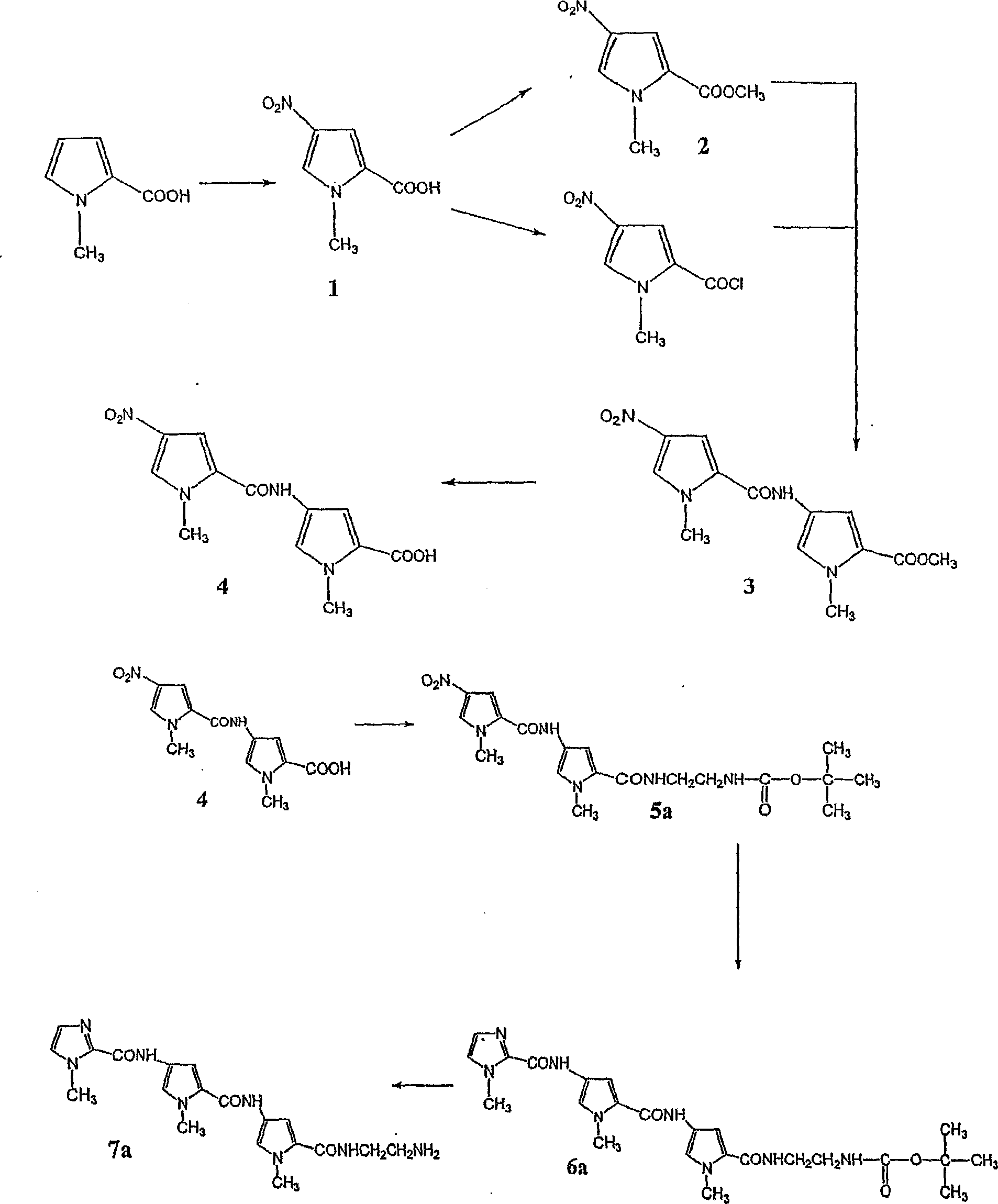
[0356] The polyamide Im / Py / Py was synthesized by a method similar to Lown et al. (J. Org. Chem., 1985, 50(20), 374-379). The synthesis diagram of Im / Py / Py is shown in figure 1 .
[0357] Methyl-4-nitropyrrole-2-carboxylic acid (1)
[0358] Heat acetic anhydride (8ml) and nitric acid (70%, 1.6mL) to 50°C, heat for 15 minutes, and cool to room temperature. Then the solution was slowly added to the Ac of 1-methyl-2-pyrrole carboxylic acid (2.0g, 0.02mol) cooled to -25°C 2 O (12ml) suspension. The mixture was stirred at -15°C for 30 minutes, warmed to room temperature, and stirring continued for 20 minutes. The mixture was cooled to -25°C again, and the resulting precipitate was collected in a funnel cooled with dry ice. Use cold Ac 2 O(-25℃) wash, then Ac 2 O: CCl 4 (1:1, -25℃) wash, then use CCl 4 Wash with hexane. The yellow solid was dissolved in NaOH (1M) and acidified with HCl to produce a microemulsion solid product, which was col...
Embodiment 2
[0378] DNA melting experiment
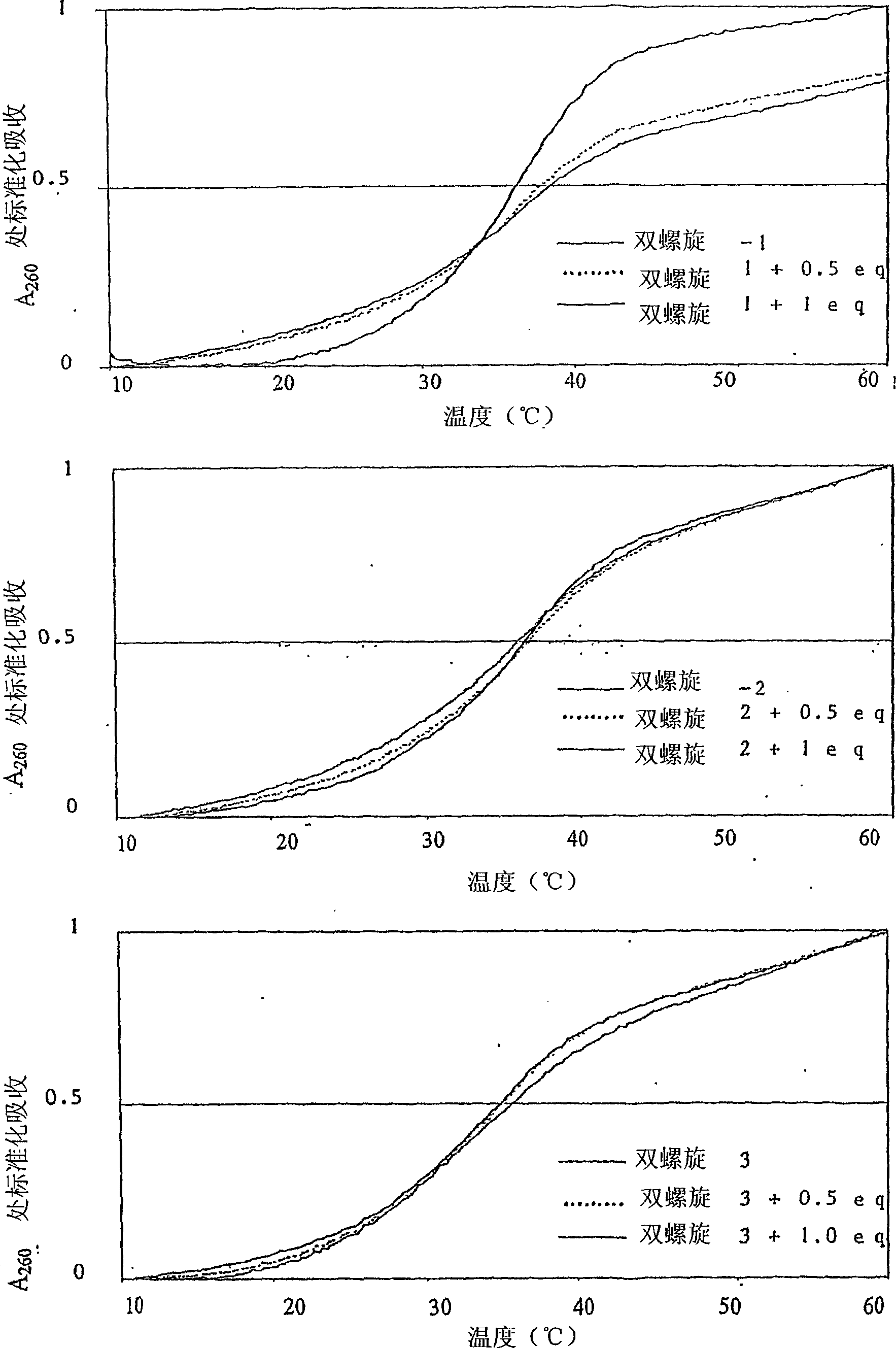
[0379] A Cary 1E recording spectrometer equipped with a Peltier controllable sample cell holder and a sample cell with a length of 1 cm was used to obtain the DNA melting curve graph of Im / Py / Py-Pt at 260 nm. The heating rate in all experiments is 0.5°C / min. The solution conditions were sodium phosphate (10mM), EDTA (1mM) and NaCl (40mM), and the pH was adjusted to 7.0. DNA melting curve such as figure 2 Shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com