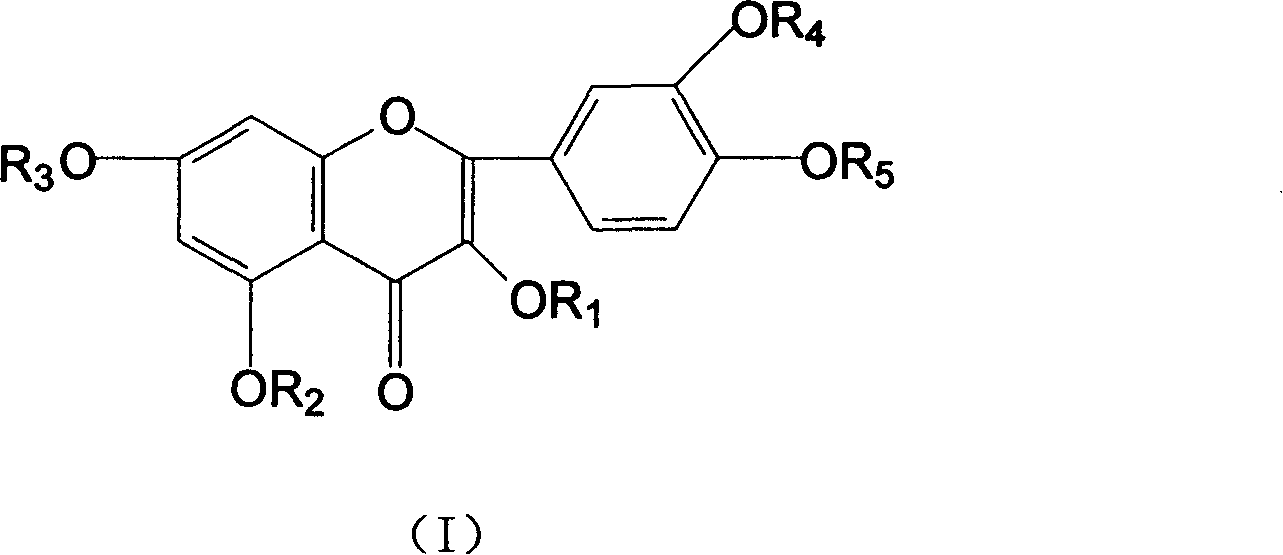
Hydroxy butyl rutin derivatives and preparation process thereof
A derivative, the technology of rutin, is applied in the field of medicine to achieve the effects of good water solubility and stability, high yield and broad market development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In a 500mL flask, add 5.5g NaOH and 250mL water, stir to dissolve. Then 45 g of rutin were added and stirred for 1.0 hour. 26 mL of 1,2-epoxybutane was added dropwise under stirring, and the reaction was continued for 30 hours. Neutralize the reaction liquid with 2mol / L hydrochloric acid to neutral, desalt with ion exchange resin, then concentrate under reduced pressure (20kPa), heat (70°C), and spray dry (not higher than 80°C) to obtain 34.6g hydroxybutyrate base rutin.
Embodiment 2
[0041]In a 250mL flask, add 2.5gNaH, 200mL DMF, stir to dissolve. Then 13 g of partially methylated rutin were added and stirred until dissolved. 7 mL of 1,2-epoxybutane was added dropwise with stirring, and the reaction was continued for 24 hours. Neutralize the reaction solution with 1mol / L sulfuric acid to neutrality, concentrate under reduced pressure, desalt and remove DMF with a gel chromatography column, and dry in vacuum (20kPa) to obtain 8.3g of methyl-rutin containing hydroxybutyl components .
Embodiment 3
[0043] In a 250mL flask, add 1.5gKOH and 100mL water, stir to dissolve. Then 6 g of partially ethylated rutin was added and stirred until dissolved. 3 mL of 2,3-epoxybutane was added dropwise with stirring, and the reaction was continued for 12 hours. Use 2mol / L acetic acid to neutralize the reaction solution to neutrality, extract the water phase through n-butanol extraction, heat the n-butanol phase under reduced pressure (5kPa) (less than 80°C) to recover n-butanol, and then vacuum-dry to obtain 4.2 g of ethyl-rutin with hydroxybutyl component.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com