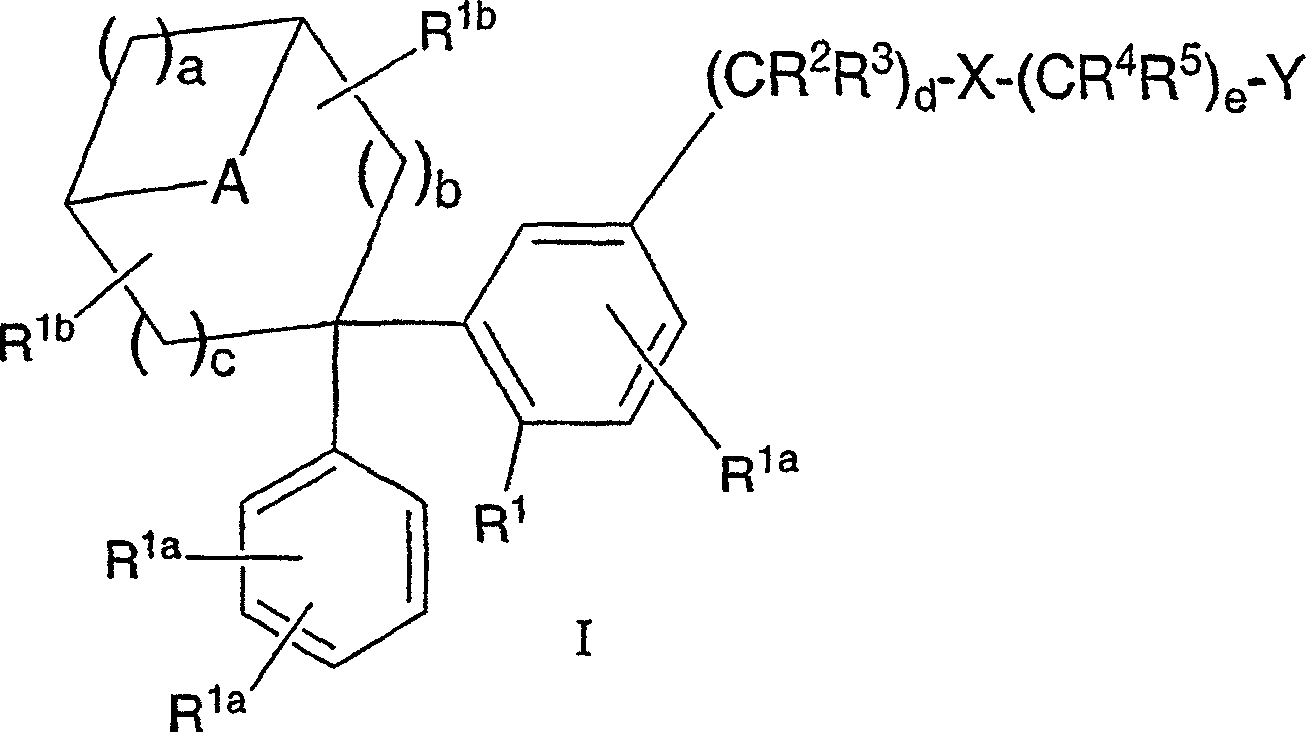
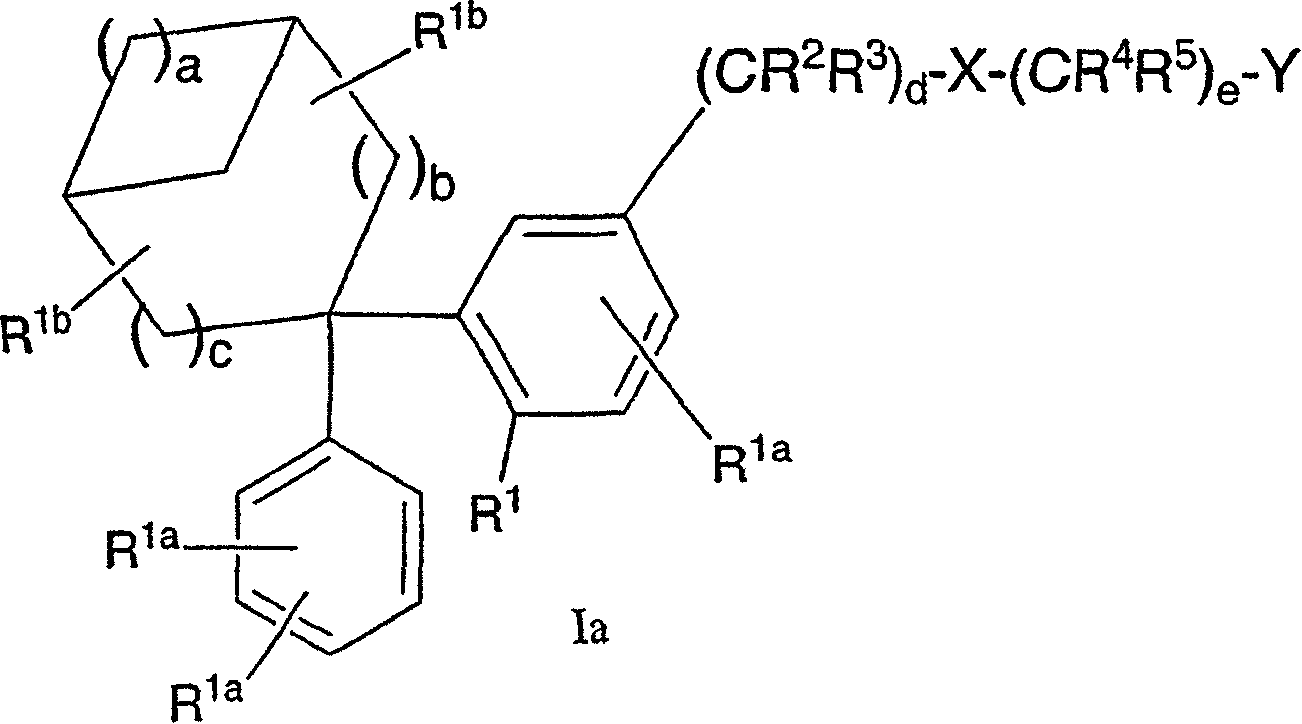
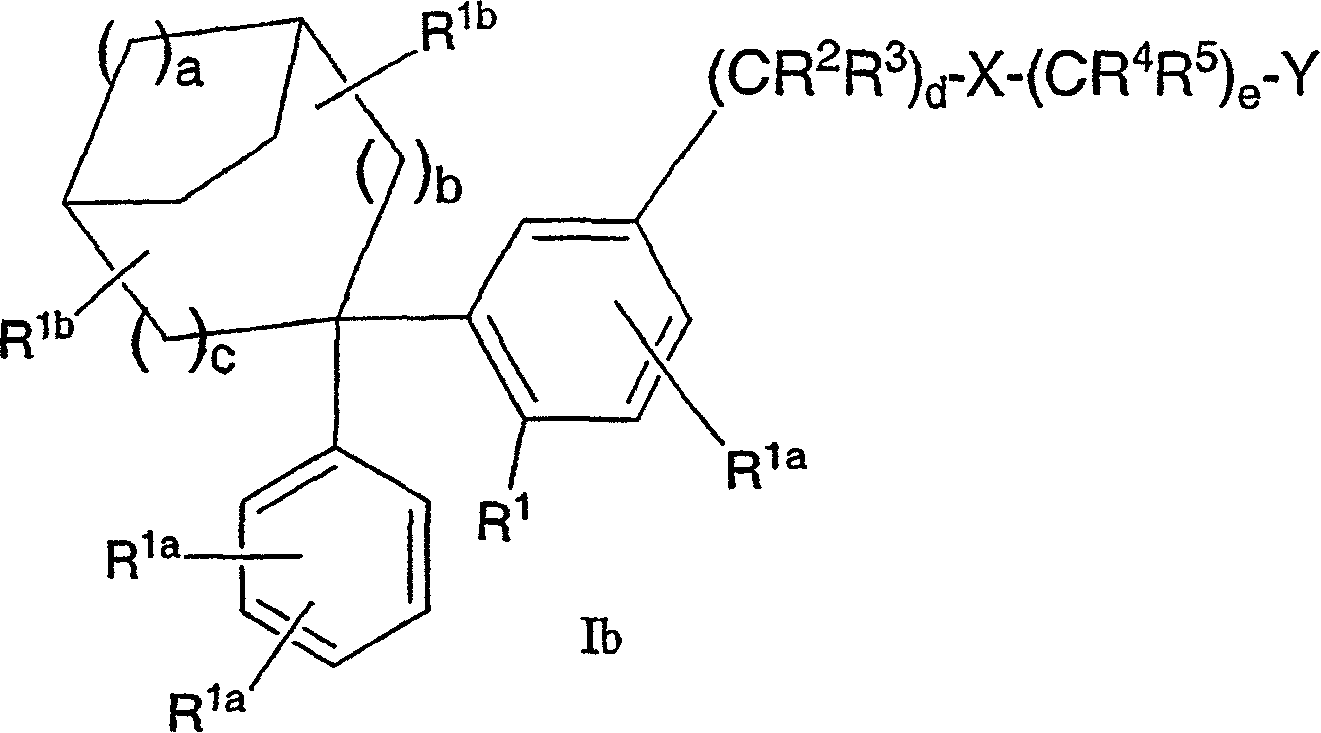
Diphenyl substituted cycloalkanes, compositions containing such compounds and methods of use
A compound, alkyl technology, used in the field of treatment and prevention of atherosclerosis and related diseases and disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0261] Preparation of intermediates:
[0262] Route I-1
[0263]
[0264] Bis[2.2.1]heptan-7-one (I-1a) was prepared according to Gassman, P.G.J.Org.Chem. 1964, 29, 160-163 and references cited therein.
[0265]Route I-2
[0266]
[0267] Preparation of (±)-2-(2-phenylbicyclo[2.2.1]hept-2-yl)benzene-1,4-diol (I-2b)
[0268] Step A: Preparation of (±)-2-phenylbicyclo[2.2.1]heptane-2-ol (I-2a)
[0269] To a solution of norcamphor (60.0 g, 0.54 mole) in THF (1 L) was added phenylmagnesium bromide (200 mL of a 3M solution in diethyl ether, 0.60 mole) at -65°C. The temperature was maintained between -65°C and -20°C during the addition. After the addition was complete, the mixture was allowed to warm to room temperature and stirred overnight. The mixture was cooled to 0°C and saturated aqueous ammonium chloride (200 mL) was carefully added. 1N Hydrochloric acid was added until the residual salt was dissolved. The mixture was then extracted twice with...
Embodiment 1a
[0286] Examples 1a, 1b and 1c
[0287] Step A v1: (±)-2-{[2-(2-Phenylbicyclo[2.2.1]hept-2-yl)-4-(quinolin-2-ylmethoxy)phenoxy]methanol Base} quinoline (1a) and (±)-2-{[2-(2-phenylbicyclo[2.2.1]hept-2-yl)-4-(quinolin-2-ylmethoxy)phenol Preparation of (1b)
[0288] Potassium tert-butoxide (23.1 mL of a 1M solution in THF, 23.1 mmol) was added dropwise to a solution of I-2b (6.48 g, 23.1 mmol) in DMF (130 mL) at room temperature. During the addition, the reaction mixture turned from heterogeneous to homogeneous. The resulting solution was left at room temperature for 20 minutes. After this time a solution of 2-(chloromethyl)quinoline (2.90 g, 22.0 mmol) in DMF (5 mL) was added and the resulting mixture was stirred at room temperature for 18 hours. The reaction mixture was poured into water / 1N hydrochloric acid (300 mL:25 mL) at 0 °C and extracted 3 times with EtOAc. The combined organic extracts were washed 3 times with water and dried (MgSO 4 ) and concen...
Embodiment 2a2bb
[0302] Example 2a.2bb 2c and 2d
[0303] Step A: (±)-2-(2-Phenylbicyclo[2.2.1]hept-2-yl)-4-(quinolin-2-ylmethoxy)phenyl triflate (2a ) preparation
[0304] A solution of 1b (500 mg, 1.19 mmol) in pyridine (5 mL) was diluted with toluene (5 mL) and cooled to 0 °C. To this mixture was added trifluoromethanesulfonic anhydride (0.20 mL, 1.19 mmol) dropwise. The resulting mixture was stirred at room temperature for 18 h. A second portion of trifluoromethanesulfonic anhydride (0.06 mL, 0.36 mmol) was added and after about 4 h, the reaction mixture was diluted with water and extracted 3 times with EtOAc. The combined organic extracts were washed with water and dried (MgSO 4 ) and concentrate. Flash chromatography (silica gel; hexane / EtOAc 9:1) of the residue gave a solid (533 mg) which was triturated with hexane to give 2a , as a solid (402 mg), m.p. 131-132°C.
[0305] Step B: Preparation of (±)-methyl 2-(2-phenylbicyclo[2.2.1]hept-2-yl)-4-(quinolin-2-ylmethoxy)benzoate (2b) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com