Use of elastic protease inhibitor for preparing medicine for protecting cerebral hemorrhage
An elastase and inhibitor technology is applied in the application field of elastase inhibitors in the preparation of cerebral hemorrhage protection drugs, and achieves the effects of high modeling success rate, good repeatability and easy positioning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
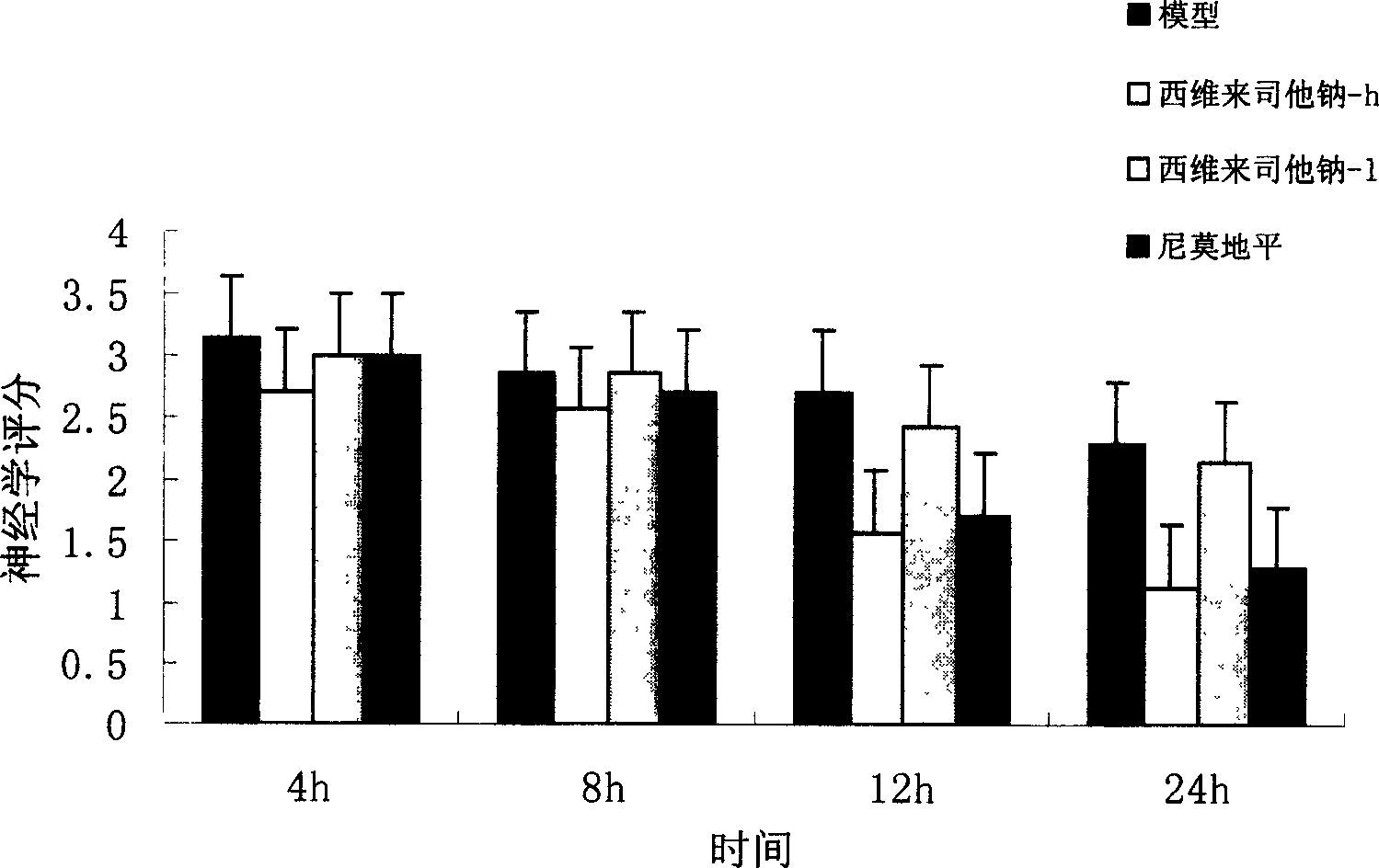
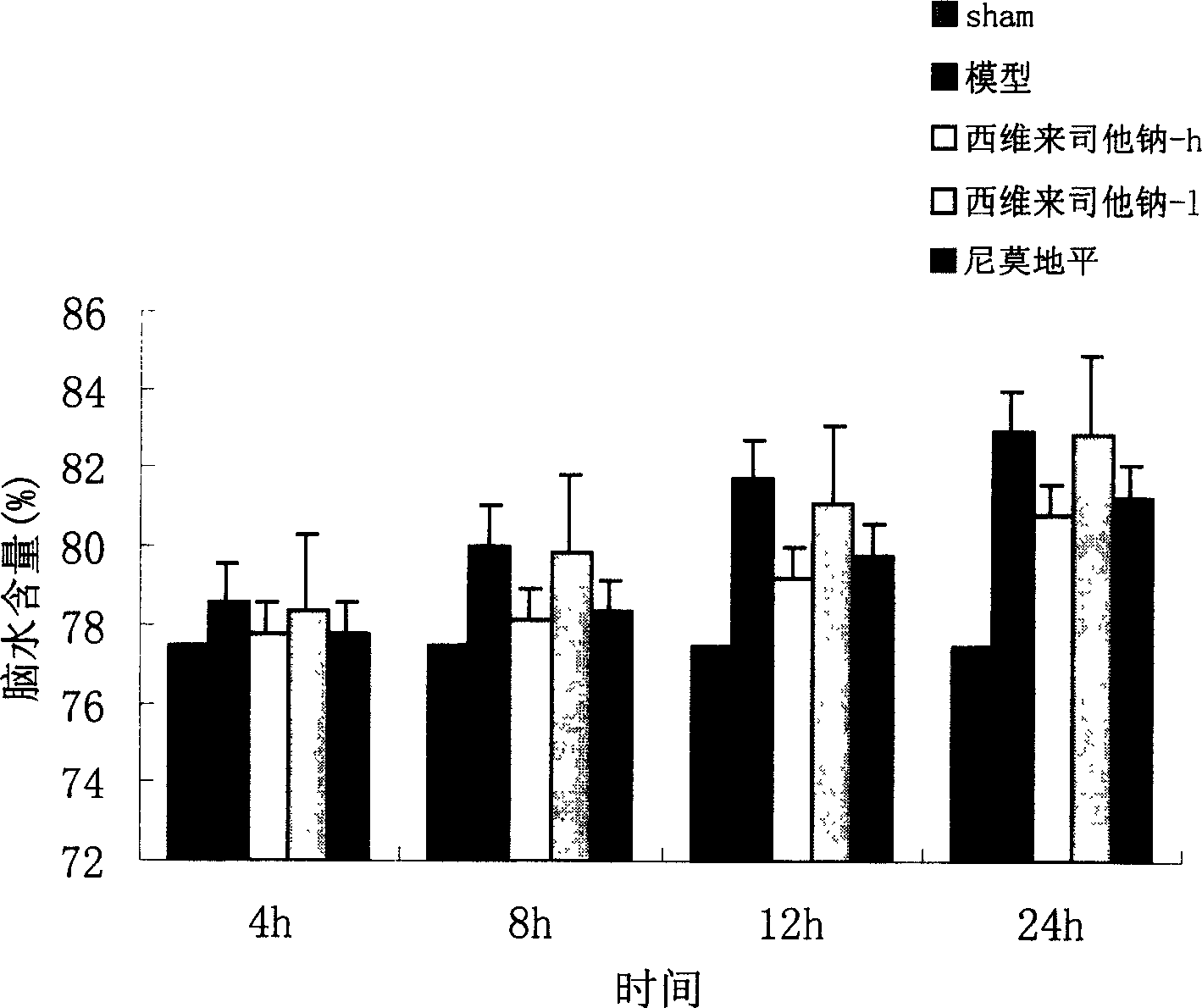
[0037] Example 1 Sivelestat sodium protects against cerebral hemorrhage in rats
[0038] Materials and methods
[0039] Experimental Materials:
[0040] Experimental animals:
[0041] Wistar rats, half male and half female, weighing 180-250 g, were provided by the Experimental Animal Center of Chongqing Medical University. 12h fasting before operation, free drinking water.
[0042] Main reagents and medicines:
[0043] Collagenase Type VII Sigma, USA
[0044] Chloral Hydrate Suzhou Zhengxing Chemical Factory
[0045] Sivelestat Sodium Chongqing Pharmaceutical Industry Research Institute Co., Ltd.
[0046] Nimodipine (Nirissu) Shandong Taian Pharmaceutical Factory
[0047] Main instruments:
[0048] Stereotaxic Instrument Northwest Optical Instrument Factory
[0049] Micro Syringe Shanghai Baoxi Glass Instrument Factory
[0050] Constant temperature drying oven Chongqing Experimental Instrument Factory
[0051] Electronic Analytical Balance Sartorius, Germany
[005...
Embodiment 2
[0091] Embodiment 2 sivelestat sodium freeze-dried powder injection
[0092] Sivelestat Sodium 100g
[0093] Mannitol 300g
[0094] Polyvinylpyrrolidone 100g
[0095] Appropriate amount of sodium carbonate
[0096] Dilute to 51 with water for injection
[0097] Makes 1000 bottles
[0098] Preparation Process:
[0099] Prepare sodium carbonate into a 4% solution for later use; mix sivelestat sodium, mannitol, and polyvinylpyrrolidone, add a certain amount of water for injection, stir well until dissolved, adjust the pH to 6.5-8.5 with sodium carbonate solution, and then Dilute to volume with water for injection, after primary filtration and sterilizing filtration, pack into 5ml / bottle, freeze-dry to obtain.
Embodiment 3
[0100] Embodiment 3 sivelestat sodium injection
[0101] Sivelestat Sodium 100g
[0102] HP-β-cyclodextrin 100g
[0103] EDTA-CaNa 1g
[0104] Sodium metabisulfite 1g
[0105] Sodium Phosphate Appropriate amount
[0106] Dilute to 5L with water for injection
[0107] Makes 1000 bottles
[0108] Preparation Process
[0109] Sodium phosphate is formulated into a 4% solution for later use; after mixing sivelestat sodium, HP-β-cyclodextrin, EDTA-CaNa, and sodium metabisulfite, add a certain amount of water for injection, stir fully to dissolve it, and then use Sodium phosphate solution is used to adjust the pH to 6.5-8.5, and water for injection is used to make up the volume, and after primary filtration and sterilizing filtration, it is obtained by subpackaging.
[0110] Instructions:
[0111] For patients with cerebral hemorrhage, the lyophilized powder injection is diluted with normal saline and infused at 0.2-0.5 mg / kg / hour, which can ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com