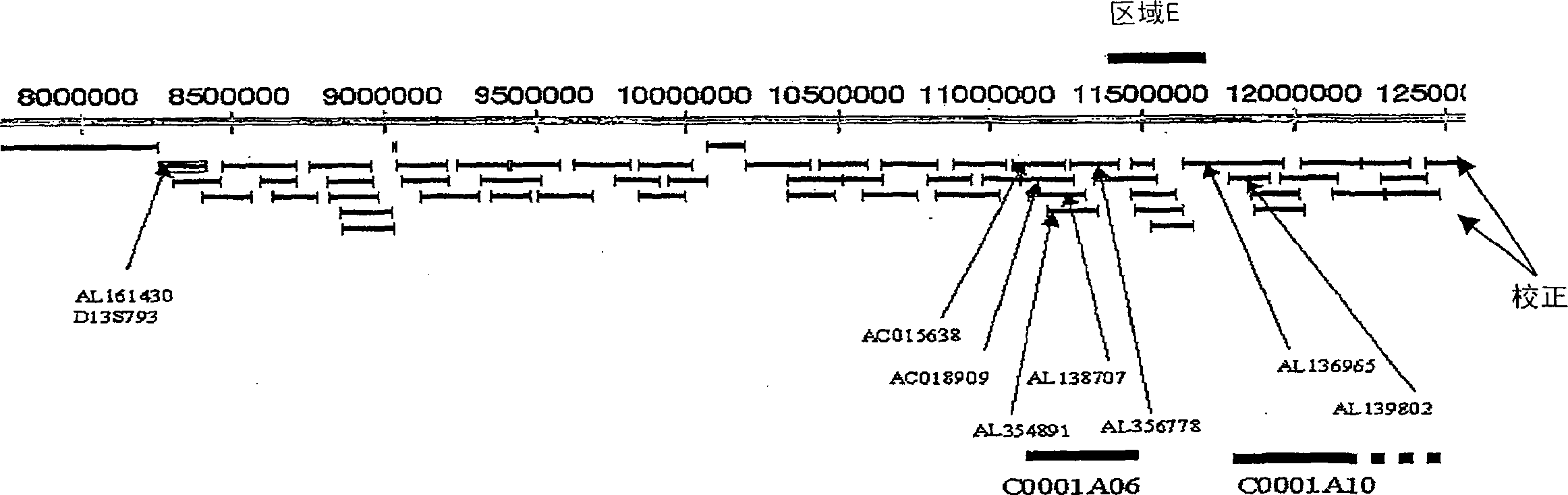
Schizophrenia-related voltage-gated ion channel gene and protein
An amino acid and polynucleotide technology, which uses in-situ gene markers to screen CanIon channel modulators, treats or prevents various diseases or symptoms, and the field of polypeptides encoded by CanIon genes can solve the problem of difficult prediction of ion channels that are specific for therapeutic intervention. disease and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
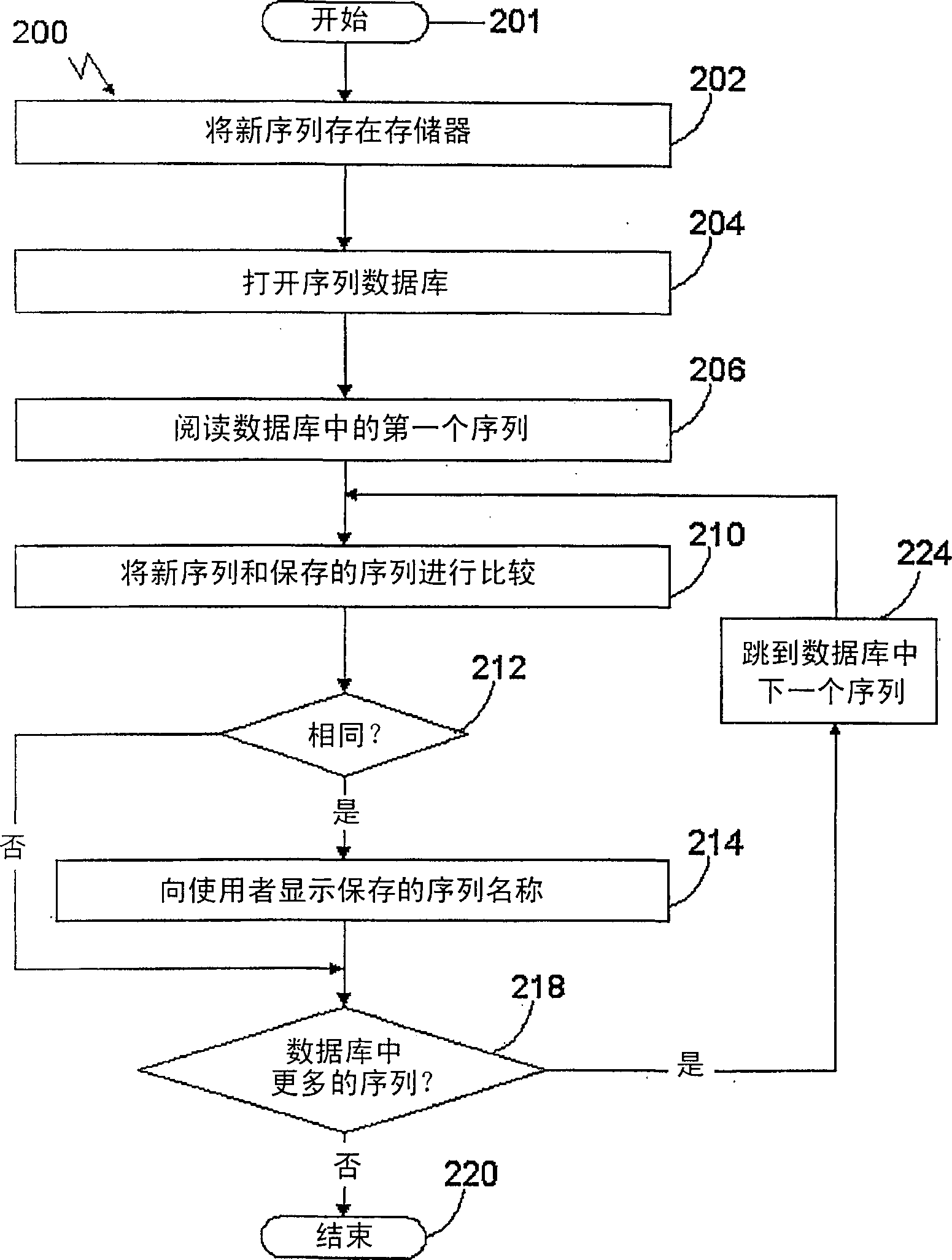
[0833] Identification of biallelic marker-DNA extracts
[0834] Donors are unrelated and healthy. They represent enough diversity to represent a heterogeneous population in France. DNA was extracted from 100 individuals and tested for biallelic markers.
[0835] 30 ml of peripheral venous blood was obtained from each donor in the presence of EDTA. 2000rpm. Cells (sediment) were collected by centrifugation for 10 minutes. With lysate (50ml final volume: 10mM Tris pH7.6, 5mM MgCl 2 , 10mM NaCl) to lyse red blood cells. After resuspending the cell pellet in lysate, the solution is centrifuged (10 minutes, 2000 rpm.) as many times as necessary to remove residual erythrocytes in the supernatant.
[0836] Lyse the leukocyte pellet overnight at 42°C with 3.7ml of lysate containing the following components:
[0837] -3ml TE 10-2 (Tris-HCl 10mM, EDTA 2mM) / NaCl 0.4M
[0838] -200 μl SDS 10%
[0839] - 500 μl K-protease (2 mg K-protease dissolved in TE 10-2 / NaCl 0.4M)
[0840...
Embodiment 2
[0845] Identification of biallelic markers: amplification of genomic DNA by PCR
[0846] Amplification of the specific genomic sequence of the DNA sample of Example 1 was performed on the DNA pool obtained above. In addition, 50 individual samples were similarly amplified.
[0847] Perform PCR assays using the following protocol:
[0848] Final volume 25μl
[0849] DNA 2ng / μl
[0850] MgCl 2 2mM
[0851] dNTP (each) 200μM
[0852] Primer (each) 2.9ng / μl
[0853] Ampli Taq Gold DNA Polymerase 0.05 unit / μl
[0854] PCR buffer (10x=0.1M trisHCl pH8.30.5M KCl) 1x
[0855] Each pair of first primers was designed using the CanIon gene sequence information disclosed herein and OSP software (Hilier & Green, 1991). This first pair of primers is approximately 20 nucleotides in length and has the sequence disclosed in Table 1 under the columns labeled PU and RP.
[0856]
In SEQ ID1 this expansion
Adder's l...
Embodiment 3
[0862] Identification of biallelic markers - testing and identification of polymorphisms on amplified genomic DNA
[0863] Sequencing of the amplified DNA obtained in Example 2 was performed on an ABI 377 sequencer. The sequence of the amplified product was determined using an automated dideoxy terminator sequencing reaction with a dye terminator cycle sequencing program. The sequencing reaction products were electrophoresed on a sequencing gel, and their sequences were determined using gel imaging analysis (ABI Prism DNA Sequencing Analysis software (version 2.1.2)).
[0864] The sequence data were further evaluated to detect the presence of biallelic markers in the amplified fragments. Polymorphism searches are based on overlapping peaks resulting from the presence of different bases at the same position above in the electrophoretic pattern.
[0865] Of the 17 fragments amplified, 18 biallelic markers were detected. The positions of these biallelic markers are shown in Ta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com